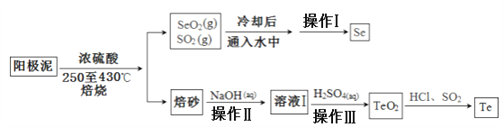
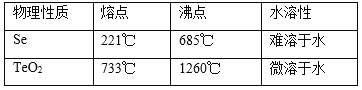
��Ŀ����
����Ŀ��A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��E�Ǿ��й�����ζ��Һ�塣A��B��C��D��E��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���

��ش��������⣺
��1����ҵ�ϣ���ʯ�ͻ��ʯ���͵ķ�����___________________��
��2����������ʯ���ͻ��A�Ĺ����е��м����֮һ������һ��ͬ���칹���к�����������![]() ����������ͬ���칹��Ľṹ��ʽ�ǣ�___________________�� D�����й����ŵ�������_______________��
����������ͬ���칹��Ľṹ��ʽ�ǣ�___________________�� D�����й����ŵ�������_______________��
��3��A��B��0.1 mol����ȫȼ������O2�������_______����״���£���
��4����ӦB��C�Ļ�ѧ����ʽΪ______________________��
��5����ӦB��D��E�Ļ�ѧ����ʽΪ______________________���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ��______________________��
���𰸡� ���� ![]() �Ȼ� 6.72L 2CH3CH2OH+O2
�Ȼ� 6.72L 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3COOH+CH3CH2OH
2CH3CHO+2H2O CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ����Ũ���������������ȵ�
CH3COOCH2CH3+H2O ����Ũ���������������ȵ�
��������A�IJ���ͨ������һ�����ҵ�ʯ�ͻ���ˮƽ����AӦΪCH2=CH2����ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ��������������
(1)��ҵ�ϣ���ʯ�ͷ�����ʯ���ͣ��ʴ�Ϊ������
(2)�����һ��ͬ���칹���к�����������������ͬ���칹��Ľṹ��ʽ�ǣ�![]() ������Ĺ��������Ȼ����ʴ�Ϊ��
������Ĺ��������Ȼ����ʴ�Ϊ��![]() ���Ȼ���
���Ȼ���
(3)��ϩ���Ҵ��ĺ�������ͬ����0.1 mol��ϩ���Ҵ���ȫȼ������O2�����ʵ���Ϊ0.1 mol��(2+![]() )=0.3mol����״���µ����Ϊ0.3mol��22.4L/mol=6.72L���ʴ�Ϊ��6.72L��
)=0.3mol����״���µ����Ϊ0.3mol��22.4L/mol=6.72L���ʴ�Ϊ��6.72L��
(4)B��C���Ҵ���Cu��Ag�����������·���������Ӧ����CH3CHO����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+H2O��
2CH3CHO+H2O��
(5)��ӦB+D��E���Ҵ���������Ũ���ᡢ��������������������������Ӧ����ʽ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O���÷�Ӧ�����ʱȽϻ�����ͨ����ȡ����Ũ���������������ȵȴ�ʩ��߸÷�Ӧ�����ʣ��ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O���÷�Ӧ�����ʱȽϻ�����ͨ����ȡ����Ũ���������������ȵȴ�ʩ��߸÷�Ӧ�����ʣ��ʴ�Ϊ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O������Ũ���������������ȵȡ�
CH3COOCH2CH3+H2O������Ũ���������������ȵȡ�

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�