��Ŀ����
 ���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵���
���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵����ڢ�
�ڢ�
������� ���ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
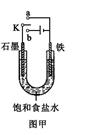
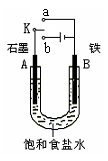
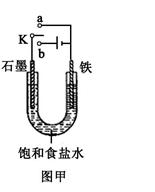
������Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ������Fe��OH��2����������ͼ��ʾʵ��װ������Ƶô�����Fe��OH��2�������������Ϸֱ�Ϊʯī������
��1��a�缫����Ϊ
Fe
Fe
���õ缫��ӦʽFe-2e-=Fe2+
Fe-2e-=Fe2+
��2������ɫ�����ڵ缫�����ɣ�����Һd��
C
C
������ɫ����������֮�����Һ�����ɣ�����Һd��B
B
������ĸ���ţ�
A����ˮ ��B��NaCl��Һ ��C��NaOH��Һ��D��CuCl2��Һ
��3��Һ��cΪ������������
������������ֹ��ɫ����������
������������ֹ��ɫ����������
��4����������֮�����Һ���ܶ�ʱ���ڿ�����ɫ���������Բ�ȡ�Ĵ�ʩ��
B
B
��A������ϡ���������Һ������B���ʵ������Դ��ѹ ����C���ʵ����͵��Һ�¶ȣ�
����������c��NaOH����V��NaOH��=c��HCl����V��HCl�������жϣ�
��1���ƴ�����Fe��OH��2��������FeΪ������ʧȥ���ӣ�a���Դ������������aΪ������
��2����ˮ������̫�Ӱ�����ʵ��Ʊ�����NaCl��NaOH��Һ�������ӷŵ磬������Fe��OH��2���������ҺΪCuCl2��Һ������Fe+CuCl2=Cu+CuCl2��
��3�������ܶ�ˮ��С��������ˮ���ɸ�����������ֹ������������������
��4����ʱ���ڿ�����ɫ����������Ӧ�����ʼ��ɣ�
��1���ƴ�����Fe��OH��2��������FeΪ������ʧȥ���ӣ�a���Դ������������aΪ������
��2����ˮ������̫�Ӱ�����ʵ��Ʊ�����NaCl��NaOH��Һ�������ӷŵ磬������Fe��OH��2���������ҺΪCuCl2��Һ������Fe+CuCl2=Cu+CuCl2��
��3�������ܶ�ˮ��С��������ˮ���ɸ�����������ֹ������������������
��4����ʱ���ڿ�����ɫ����������Ӧ�����ʼ��ɣ�
�����ʽ�ζ���������ˮϴ����Ӧ�ñ�Һ��ϴ��ϴ������ᵼ������ƿ�����ô���Һ��ϴ���ζ�ǰӦ�ų��ζ����е����ݣ���٢ܴۢ��ڢ���ȷ���ʴ�Ϊ���ڢݣ�
��1���ƴ�����Fe��OH��2��������FeΪ������ʧȥ���ӣ�a���Դ������������aΪ�����������ĵ缫��ӦΪFe-2e-=Fe2+���ʴ�Ϊ��Fe��Fe-2e-=Fe2+��
��2����ˮ������̫�Ӱ�����ʵ��Ʊ�����NaCl��NaOH��Һ�������ӷŵ磬������Fe��OH��2���������ҺΪCuCl2��Һ������Fe+CuCl2=Cu+CuCl2������ɫ�����ڵ缫�����ɣ�Ӧѡ��NaOHΪ�������Һ������ɫ����������֮�����Һ�����ɣ�Ӧѡ��NaCl��������ɵ����������Ӻ�ͭ���ӷֱ��������ƶ������м�����ɳ�����
�ʴ�Ϊ��C��B��
��3�������ܶ�ˮ��С��������ˮ���ɸ�����������ֹ������������������Ϊ��ֹ����������������������ʵ����뱽֮ǰ����d��Һ���м��ȴ�����Ŀ�����ų���Һ�е�������
���ʴ�Ϊ��������������ֹ��ɫ������������
��4����ʱ���ڿ�����ɫ�������ʵ������Դ��ѹ���ʵ���С���缫����������Ӧ�����ʣ�������ϡ��������ɳ����������¶ȷ�Ӧ���ʼ������ʴ�Ϊ��B��
��1���ƴ�����Fe��OH��2��������FeΪ������ʧȥ���ӣ�a���Դ������������aΪ�����������ĵ缫��ӦΪFe-2e-=Fe2+���ʴ�Ϊ��Fe��Fe-2e-=Fe2+��
��2����ˮ������̫�Ӱ�����ʵ��Ʊ�����NaCl��NaOH��Һ�������ӷŵ磬������Fe��OH��2���������ҺΪCuCl2��Һ������Fe+CuCl2=Cu+CuCl2������ɫ�����ڵ缫�����ɣ�Ӧѡ��NaOHΪ�������Һ������ɫ����������֮�����Һ�����ɣ�Ӧѡ��NaCl��������ɵ����������Ӻ�ͭ���ӷֱ��������ƶ������м�����ɳ�����
�ʴ�Ϊ��C��B��
��3�������ܶ�ˮ��С��������ˮ���ɸ�����������ֹ������������������Ϊ��ֹ����������������������ʵ����뱽֮ǰ����d��Һ���м��ȴ�����Ŀ�����ų���Һ�е�������
���ʴ�Ϊ��������������ֹ��ɫ������������
��4����ʱ���ڿ�����ɫ�������ʵ������Դ��ѹ���ʵ���С���缫����������Ӧ�����ʣ�������ϡ��������ɳ����������¶ȷ�Ӧ���ʼ������ʴ�Ϊ��B��
�����������ۺϿ����к͵ζ����������������Ʊ��͵��ԭ������ȷ���ԭ����FeΪ�����ǽ����Ĺؼ���ע��ʵ���з�ֹ��������������������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
�����Ŀ
 2PbSO4+2H2O
2PbSO4+2H2O 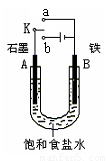
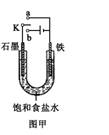 ������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е�
��ʴ��
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е�
��ʴ��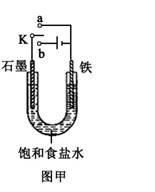
 2PbSO4+2H2O
2PbSO4+2H2O
 ������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��