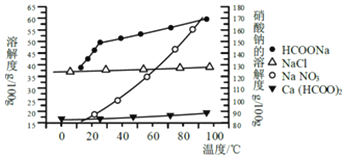
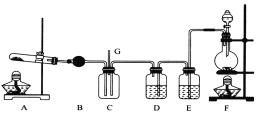
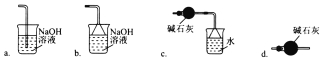
��Ŀ����
����Ŀ��Ԫ��A��D��Ԫ�����ڱ��ж����ڵ�����Ԫ�أ�����ݱ�����Ϣ�ش���������
Ԫ�� | ���ʻ�ṹ��Ϣ |
A | �����Ƴɵĸ�ѹ�ƣ������Ļƹ�����ǿ�����Զ�����䵥��ȼ�պ����ɵ���ɫ���� |
B | ��ҵ��ͨ������Һ̬��������䵥�ʡ�ԭ�ӵ��������������ڲ����������2.5�� |
C | ������˫ԭ�ӷ��ӣ����¡���ѹ���ǵ�����ɫ���壬ԭ�ӵ�L��������һ�����Ӽ��ﵽ�ȶ��ṹ |
D | +2�������ӵĺ�������Ų�����ԭ����ͬ |
��1���ϱ�����A����ͬһ���ڵ�Ԫ���ǣ�дԪ�ط��ţ�_____ ��
����D���ӵĽṹʾ��ͼ__________________ ��
��2����֪C�ĵ�������H2O�����û���Ӧ����O2��д��C������ˮ��Ӧ�Ļ�ѧ����ʽ_ ��
��3����Ԫ��B�ĵ��ʻ���������ȷ���� _��
a��BԪ�ص��������Ϊ+6 b�����¡���ѹ�µ���������ˮ
c�����ʷ����к���18������ d����һ���������������뵥��B��Ӧ
��4��A��D��Ԫ�ػ����Խ�ǿ���ǣ�дԪ�����ƣ� _____ ��
���𰸡���1��Mg��![]() ����2��2F2+2H2O=4HF+O2��3��b��d��4����
����2��2F2+2H2O=4HF+O2��3��b��d��4����
������������������ɱ�����Ϣ��֪��A����Ԫ�أ�B�ǵ�Ԫ�أ�C�Ƿ�Ԫ�أ�D��þԪ�أ�
��1��A����Ԫ�أ�λ�ڵ������ڣ����ϱ�����A����ͬһ���ڵ�Ԫ����Mg�����ӽṹʾ��ͼΪ![]() ��
��
��2��C�ĵ�������H2O�����û���Ӧ����O2��C������ˮ��Ӧ�Ļ�ѧ����ʽΪ2F2+2H2O=4HF+O2��
��3��A��NԪ�ص��������Ϊ+5������b�����¡���ѹ�µ���������ˮ����ȷ��c�����ʷ����к�14�����ӣ�����d����һ���������������뵪����Ӧ���ɰ�������ȷ����ѡbd��
��4��ͬһ���ڴ����ң���������������A��D��Ԫ�ػ����Խ�ǿ�����ơ�

����Ŀ���±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������
ϩ����� | ������� |
(CH3)2C=CHCH3 | 10.4 |
CH3CH=CH2 | 2.03 |
CH2=CH2 | 1.00 |
CH2=CHBr | 0.04 |
��1�����л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ������й������ƣ����з�Ӧ����������______������ţ���
A����CH3��2C=C(CH3)2 B��CH3CH=CHCH2CH3
C��CH2=CH CH3 D��CH2=CHCl
��2��ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
CH2=CHCH3+HBr��CH3CHBrCH3+CH3CH2Br
����Ҫ�������Ҫ���
��д�� ��HBr��Ӧ����Ҫ����Ľṹ��ʽ_____________��
��HBr��Ӧ����Ҫ����Ľṹ��ʽ_____________��
����Ŀ�����Ŵ�����Ⱦ���������أ�����������ʮ�������ڼ䣬����������SO2���ŷ�������8%���������NOx���ŷ�������10%��������̼��CO2�����ŷ���ҲҪ������١�
��1���ں��£��ݻ�Ϊ1 L�����У�����Է�������ת�����䷴Ӧ���̺�������ϵ��ͼ1��ʾ(��֪��2SO2(g)��O2(g) ![]() 2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺

��д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��_________________________________��
����H2��__________kJ��mol��1��
������ͬ�����£�����1 mol SO3��0.5 mol��O2����ﵽƽ��ʱSO3��ת����Ϊ______________����ʱ�÷�Ӧ________(��ų��������ա�)________kJ��������
��2���й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��50%��
��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�Ӧ��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ2��ʾ����3 min��9 min��v(H2)��________mol��L��1��min��1��
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1�����CO2��CH3OH(g)Ũ����ʱ��仯��ͼ2��ʾ����3 min��9 min��v(H2)��________mol��L��1��min��1��
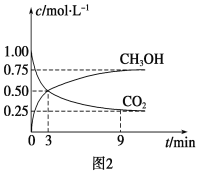
����˵��������Ӧ�ﵽƽ��״̬����________(����)��
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1(��ͼ�н����)
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ��������3 mol H2��ͬʱ����1 mol H2O
D��CO2����������ڻ�������б��ֲ���
��Ϊ�˼ӿ컯ѧ��Ӧ������ʹ��ϵ����������ʵ������٣�������������ʱ���ɲ�ȡ�Ĵ�ʩ��________(����)��
A�������¶� B����С������� C���ٳ���CO2���� D��ʹ�ú��ʵĴ���
��3����ҵ�ϣ�CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO(g)��2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��������˵����ȷ����________��
CH3OH(g)��ƽ�ⳣ��������˵����ȷ����________��
�¶�/�� | 0 | 100 | 200 | 300 | 400 |
ƽ�ⳣ�� | 667 | 13 | 1.9��10��2 | 2.4��10��4 | 1��10��5 |
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B���÷�Ӧ�ڵ����²����Է����У������¿��Է����У�˵���÷�Ӧ��S<0
C����T ��ʱ��1 L�ܱ������У�Ͷ��0.1 mol CO��0.2 mol H2���ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D����ҵ�ϲ����Ըߵ�ѹǿ(5 MPa)��250 ��������Ϊ�������£�ԭ����ת�������
����Ŀ����1����HNO3�������Ӧ������ֶ�����Ϊ̽��HNO3���ʣ�ij��ȤС��������̽������Ũ�����ˮ���ղ�ͬ�������ɲ�ͬŨ�ȵ�������Һ����ȡ10mL������Һ�ֱ���ͭƬ��Ӧ��ʵ���¼���£�
��� | Ũ������ˮ������� | ʵ �� �� �� |
�� | 1 �s1 | ��Ӧ���ʿ죬��Һ�ܿ�����ɫ��ͭ˿�����д�������ð��������ʺ���ɫ |
�� | 1 �s3 | ��Ӧ���ʽϿ죬��Һ�����ɫ��ͭ˿�����д�������ð����������ɫ |
�� | 1 �s5 | ��Ӧ���������Ⱥ����ʼӿ죬��Һ�����ɫ��ͭ˿����������ð����������ɫ |
�Ʊ�NO���������˵��ǣ� �������ǣ� ��
��2��.��ȤС����ľ̿��Ũ����Ϊ��ʼԭ�ϣ�̽��һ��������������Ʒ�Ӧ�Ʊ��������ơ����װ������(����װ���п�����Ӱ��)����ش��������⣺
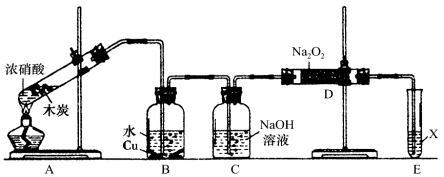
����װ������������е�һ������ǣ�_________________��
���Ʋ�B�п��Թ۲쵽����Ҫ�����ǣ�______��Cװ�õ������ǣ� ��
��װ��D�г�����NaNO2�⣬������һ�ֹ�̬����Y��Y�Ļ�ѧʽ�ǣ�_____������ͨ���ʵ��Ľ���������Y���ʣ���������Ľ������� _��
��Eװ�õ�ʵ��Ŀ���ǣ� ��