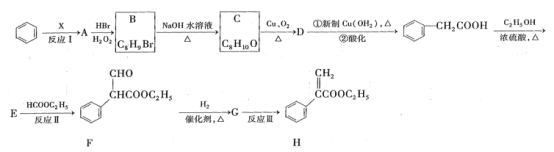
��Ŀ����
����Ŀ��(1) ��ͼ��ʾ�Ƿ�������ʱ���õ��������ش��������⣺
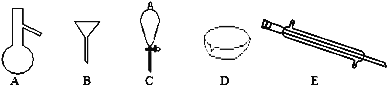
�������C��E������________________��________________��
�ڷ������»����Ӧ����Ҫѡ������ʲô����(����ĸ����)
a�����κ���ɳ��________ b�������ͺ�ˮ___________��
(2)�������ʵ���״����ͽ�����࣬����Ӧ����___________��
���� ���ܵ���Ĵ����� �ۺ����� �ܻ���� �ݻ����� ��Ԫ�� �ߵ����
A���٢ڢۢܢ� B���٢ۢݢޢ� C���٢ڢۢݢޢ� D���ڢۢݢޢ�
(3)��Fe(OH)3���������������3mol/L���������������_______________________��
(4)����������10�����ʣ��� H2O �ڿ��� ��Mg �� NH3 �� ϡH2SO4�� Ca��OH��2 ��CuSO4 .5 H2O ���Ƣ���ˮ�Ҵ���NaHCO3���У�����ˮ��Һ�еĵ��뷽��ʽΪ______�����ڵ���ʵ���__________ ������ţ���ͬ�������ڷǵ���ʵ���__________ �����ڼȲ��ǵ����Ҳ���Ƿǵ���ʵ���__________ ��
���𰸡���Һ©�� �����ܣ�ֱ�������ܣ� B C B �Ȳ������ɫ���������ܽ⣨��Һ��Ϊ�ػ�ɫ�� H2SO4=2H����SO42- �٢ޢߢ� �ܢ� �ڢۢݢ�
��������
(1) �ٸ����������ص㣬CΪ��Һ©����EΪֱ�������ܣ�
��a�����ο�����ˮ����ɳ������ˮ�����ù��˵ķ������룻
b�������ͺ�ˮΪ�������ܵ�Һ̬����
(2) �������ڶ�Ԫǿ�ᣬ�����к�����ԭ��Ϊ�����ᣬΪ���ۻ����ﲻ�ܵ��磬Ϊ�������ˮ��Һ�ܵ��磬���ڵ���ʣ�
(3)���ݽ�������ʻش�
(4) �� H2O�������룬Ϊ������ʣ�
�ڿ���Ϊ�����Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
��MgΪ���ʣ��Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
�� NH3��ˮ��Һ��Ȼ�ܵ��磬����������Ӳ���NH3����ģ�NH3���ڻ����Ϊ�ǵ���ʣ�
�� ϡH2SO4Ϊ�����Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
�� Ca��OH��2��ˮ��Һ�ܵ��磬Ϊ����ʣ�
��CuSO4 .5 H2O��ˮ��Һ�ܵ��磬Ϊ����ʣ�
����Ϊ�����Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
����ˮ�Ҵ����ڻ����ˮ��Һ��Һ̬�������磬Ϊ�ǵ���ʣ�
��NaHCO3��ˮ��Һ�ܵ��磬Ϊ����ʡ�
(1) �ٸ����������ص㣬CΪ��Һ©����EΪֱ�������ܣ�
��a�����ο�����ˮ����ɳ������ˮ�����ù��˵ķ������룬ѡ��©��B��
b�������ͺ�ˮΪ�������ܵ�Һ̬�������÷�Һ©��C���룻
(2) �������ڶ�Ԫǿ�ᣬ�����к�����ԭ��Ϊ�����ᣬΪ���ۻ����ﲻ�ܵ��磬Ϊ�������ˮ��Һ�ܵ��磬���ڵ���ʣ�������������ΪB��
(3)����Ϊ����ʣ�����Fe(OH)3�����У������Ⱦ۳��������������������������������������ᷴӦ������������Һ����������ܽ�õ��ػ�ɫ��Һ��
(4) �� H2O�������룬Ϊ������ʣ�
�ڿ���Ϊ�����Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
��MgΪ���ʣ��Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
�� NH3��ˮ��Һ��Ȼ�ܵ��磬����������Ӳ���NH3����ģ�NH3Ϊ�ǵ���ʣ�
�� ϡH2SO4Ϊ�����Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
�� Ca��OH��2��ˮ��Һ�ܵ��磬Ϊ����ʣ�
��CuSO4 .5 H2O��ˮ��Һ�ܵ��磬Ϊ����ʣ�
����Ϊ�����Ȳ��ǵ���ʣ�Ҳ���Ƿǵ���ʣ�
����ˮ�Ҵ����ڻ����ˮ��Һ��Һ̬�������磬Ϊ�ǵ���ʣ�
��NaHCO3��ˮ��Һ�ܵ��磬Ϊ����ʣ�
�������������ڵ���ʵ��Ǣ١��ޡ��ߡ��⣻���ڷǵ���ʵ��Ǣܡ�����ڼȲ��ǵ����Ҳ���Ƿǵ���ʵ��Ǣڡ��ۡ��ݡ��ࣻ����Ϊ��Ԫǿ�ᣬ��ˮ����ȫ���룬���뷽��ʽΪH2SO4=2H����SO42-��

����Ŀ��ʵ�����û�������ˮ�ķ����ϳɻ���ϩ����ʵ���װ������ͼ��ʾ��

�����õ����й��������£�
��Է������� | �ܶȣ�(g��cm-3) | �е㣯�� | �ܽ��� | |
���Ѵ� | 100 | 0.9618 | 161 | ����ˮ |
����ϩ | 82 | 0.8102 | 83 | ������ˮ |
������ʵ�鲽��ش����⣺
��������ϳ�
��a�м���10.0g��������2Ƭ���Ƭ����ȴ��������������1mLŨ���ᣬb��ͨ����ȴˮ��ʼ��������a�������������¶Ƚӽ�90����
��l�����Ƭ��������________��b��������________��
��2��a�з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________________����ʵ�������ײ������л�������Ľṹ��ʽΪ________��
���������ᴿ
����Ӧ�ֲ��ﵹ���Һ©���У�����������5%̼������Һ��ˮϴ�ӣ�����������ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ƣ�����ͨ������X�õ������Ļ���ϩ��������������Ϊ4.1g��
��3����̼������Һϴ�ӵ�������________������X������Ϊ________��
����������������ʼ���
��4���ٺ˴Ź��������������������Ƿ�Ϊ����ϩ������ϩ��������_______�ֲ�ͬ��ѧ��������ԭ�ӡ�
�� ��ʵ�����û���ϩ�IJ�����_______��
����Ŀ���¶�ΪTʱ����2.0L�����ܱ������г���1.0 mol PCl5����ӦPCl5(g)![]() PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ����
PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ����
t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3)/ mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
A����Ӧ��ǰ50 s��ƽ������Ϊv(PCl3)=0.0032mol��L��1��s��1
B�����������������䣬�����¶ȣ�ƽ��ʱc(PCl3)=0.11mol��L��1����Ӧ����H��0
C����ͬ�¶��£���ʼʱ�������г���1.0mol PCl5��0.20mol PCl3��0.20molCl2���ﵽƽ��ǰv(��)��v(��)
D����ͬ�¶��£���ʼʱ�������г���2.0mol PCl3��2.0molCl2���ﵽƽ��ʱ��PCl3��ת����С��80%
����Ŀ����1����������ԭ��Ӧ�У�������__���ӣ������ķ�Ӧ��__��Ӧ����ԭ��__���ӣ������ķ�Ӧ��__��Ӧ������������Ӧ�ķ���ʽΪ2Fe+3Cl2![]() 2FeCl3��������������__�ۣ���������ķ�ӦʽΪ___��������������__�ۣ���һ��ʵ֤���������������Ա������������(����ǿ��������)___��
2FeCl3��������������__�ۣ���������ķ�ӦʽΪ___��������������__�ۣ���һ��ʵ֤���������������Ա������������(����ǿ��������)___��
��2��0.6mol��������0.4mol����O3����֮��Ϊ___�����Ӹ���֮��Ϊ___����ԭ�Ӹ���֮��Ϊ___��
��3������һƿA��B�Ļ��Һ����֪���ǵ��������±���
���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
A | ��11.5 | 198 | 1.11 | A��B���ܣ��Ҿ�������ˮ |
B | 17.9 | 290 | 1.26 |
�ݴ˷�������A��B�����ij��÷����ǣ�___��
��4������������Na+��K+��Cu2+��H+��NO3����Cl����CO32����OH�������ܴ���������ͬһ��Һ������������Ƿֳ�A��B���飬����ÿ���о������������Ӻ����������ӡ�
A�飺___��B�飺____��