��Ŀ����
��2013?������һģ��I�����ǻ��������һ��ǿЧ�ĵ�����ϣ���������Һ����ʾ����ҵ��Ӧ�ù㷺���ṹ��ʽ��ͼ��
��1�����л���ķ���ʽΪ
��2�����л����ܷ����ķ�Ӧ�����ǣ���д���ţ�
A��������Ӧ B����ȥ��Ӧ C���Ӿ۷�Ӧ D��ˮ�ⷴӦ
���ܻ����ֳ����ܼ�����һ�����Ӳ��ϵ������Ի�ʹ����Һ�������Ӽ������ܼ�DEHP��C24H38O4����ͨ�����������Ʊ�������A����������6��̼ԭ�ӣ�D�ǶԶ��ױ���һ��ͬ���칹�壬E�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ�

�ش��������⣺
��1��E�Ľṹ��ʽ��

��2��B��C�ķ�Ӧ������
��3��3-�������ijͬ���칹��˴Ź���������ʾֻ��һ���⣬д����ͬ���칹��Ľṹ��ʽ��������
�ٽṹ��ʽ
��4��DEHP�Ľṹ��ʽ��

��5��F��E��һ��ͬ���칹�壬��������������
a���DZ�����λ��ȡ���
b����FeCl3��Һ����ɫ��
c������̼��������Һ��Ӧ��
д��F��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ��
 ��
��

��1�����л���ķ���ʽΪ
C9H8O3
C9H8O3
���京�������ŵ����������ǻ����Ȼ�
���ǻ����Ȼ�
����2�����л����ܷ����ķ�Ӧ�����ǣ���д���ţ�
AC
AC
��A��������Ӧ B����ȥ��Ӧ C���Ӿ۷�Ӧ D��ˮ�ⷴӦ
���ܻ����ֳ����ܼ�����һ�����Ӳ��ϵ������Ի�ʹ����Һ�������Ӽ������ܼ�DEHP��C24H38O4����ͨ�����������Ʊ�������A����������6��̼ԭ�ӣ�D�ǶԶ��ױ���һ��ͬ���칹�壬E�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ�

�ش��������⣺
��1��E�Ľṹ��ʽ��


��2��B��C�ķ�Ӧ������
ȡ����Ӧ��ˮ�ⷴӦ
ȡ����Ӧ��ˮ�ⷴӦ
��3��3-�������ijͬ���칹��˴Ź���������ʾֻ��һ���⣬д����ͬ���칹��Ľṹ��ʽ��������
�ٽṹ��ʽ
��CH3��3CC��CH3��3
��CH3��3CC��CH3��3
������2��2��3��3-�ļ�����
2��2��3��3-�ļ�����
��4��DEHP�Ľṹ��ʽ��


��5��F��E��һ��ͬ���칹�壬��������������
a���DZ�����λ��ȡ���
b����FeCl3��Һ����ɫ��
c������̼��������Һ��Ӧ��
д��F��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ��


��������1�����ݶ��ǻ������Ľṹ��ʽ��д�����ʽ������ΪCԭ�ӣ�����Hԭ�ӱ���C���ļ۽ṹ��
�ɽṹ��ʽ��֪�����з��ǻ����Ȼ���̼̼˫�����ֹ����ţ�
��2�����л��ﺬ�з��ǻ������зӵ����ʣ������Ȼ���������������ʣ�����̼̼˫��������ϩ�������ʣ��ݴ˽��ѡ���жϣ�������̼̼˫�����������������ӳɷ�Ӧ��
II��A�����������ӳɷ�Ӧ����3-�����飬A����������6��̼ԭ�ӣ���A�Ľṹ��ʽΪ��CH2=C��CH2CH3��CH2CH2CH2CH3��A���廯�ⷢ���ӳɷ�Ӧ����B�����������Ϣ֪��B�Ľṹ��ʽΪ��CH2BrCH��CH2CH3��CH2CH2CH2CH3��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��C�Ľṹ��ʽΪ��CH2OHCH��CH2CH3��CH2CH2CH2CH3��D�ǶԶ��ױ���һ��ͬ���칹�壬D�����Ը��������������E�ᣬE�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ���D���ڶ��ױ���E�Ľṹ��ʽΪ�� ��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ��
��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ�� �������л�������ʷ������
�������л�������ʷ������
�ɽṹ��ʽ��֪�����з��ǻ����Ȼ���̼̼˫�����ֹ����ţ�
��2�����л��ﺬ�з��ǻ������зӵ����ʣ������Ȼ���������������ʣ�����̼̼˫��������ϩ�������ʣ��ݴ˽��ѡ���жϣ�������̼̼˫�����������������ӳɷ�Ӧ��
II��A�����������ӳɷ�Ӧ����3-�����飬A����������6��̼ԭ�ӣ���A�Ľṹ��ʽΪ��CH2=C��CH2CH3��CH2CH2CH2CH3��A���廯�ⷢ���ӳɷ�Ӧ����B�����������Ϣ֪��B�Ľṹ��ʽΪ��CH2BrCH��CH2CH3��CH2CH2CH2CH3��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��C�Ľṹ��ʽΪ��CH2OHCH��CH2CH3��CH2CH2CH2CH3��D�ǶԶ��ױ���һ��ͬ���칹�壬D�����Ը��������������E�ᣬE�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ���D���ڶ��ױ���E�Ľṹ��ʽΪ��
 ��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ��
��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ�� �������л�������ʷ������
�������л�������ʷ����������⣺��1�����ݶ��ǻ������Ľṹ��ʽ��֪�������ʽΪC9H8O3���ɽṹ��ʽ��֪�������ǻ����Ȼ���̼̼˫�����ֹ����ţ���ֻ�з��ǻ����Ȼ�������ԭ�ӣ��ʴ�Ϊ��C9H8O3�����ǻ����Ȼ���
��2�����л��ﺬ�з��ǻ�������̼̼˫�����ܷ���������Ӧ������̼̼˫�������Է����Ӿ۷�Ӧ�����ܷ�����ȥ��Ӧ��ˮ�ⷴӦ���ʴ�Ϊ��AC��
II��A�����������ӳɷ�Ӧ����3-�����飬A����������6��̼ԭ�ӣ���A�Ľṹ��ʽΪ��CH2=C��CH2CH3��CH2CH2CH2CH3��A���廯�ⷢ���ӳɷ�Ӧ����B�����������Ϣ֪��B�Ľṹ��ʽΪ��CH2BrCH��CH2CH3��CH2CH2CH2CH3��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��C�Ľṹ��ʽΪ��CH2OHCH��CH2CH3��CH2CH2CH2CH3��D�ǶԶ��ױ���һ��ͬ���칹�壬D�����Ը��������������E�ᣬE�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ���D���ڶ��ױ���E�Ľṹ��ʽΪ�� ��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ��
��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ�� ��
��
��1��ͨ�����Ϸ���֪��E�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����ˮ�ⷴӦ������C������B��C�ķ�Ӧ������ȡ����Ӧ��ˮ�ⷴӦ��
�ʴ�Ϊ��ȡ����Ӧ��ˮ�ⷴӦ��
��3��3-�������ijͬ���칹��˴Ź���������ʾֻ��һ���⣬˵����������ֻ��һ�����͵���ԭ�ӣ�������ṹ��ʽΪ����CH3��3CC��CH3��3��������Ϊ��2��2��3��3-�ļ����飬
�ʴ�Ϊ����CH3��3CC��CH3��3��2��2��3��3-�ļ����飻
��4��ͨ�����Ϸ���֪��DEHP�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5��F�Ľṹ��ʽ�з�������������F�DZ�����λ��ȡ���˵��F�к��б�������ȡ��λ��Ϊ�����ϵ���λ����FeCl3��Һ����ɫ��˵��F�к��з��ǻ���F����̼��������Һ��Ӧ��˵��F�����Ȼ�������F�Ľṹ��ʽΪ��
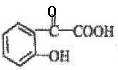 ��F��̼��������Һ��Ӧ�ķ���ʽΪ��
��F��̼��������Һ��Ӧ�ķ���ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2�����л��ﺬ�з��ǻ�������̼̼˫�����ܷ���������Ӧ������̼̼˫�������Է����Ӿ۷�Ӧ�����ܷ�����ȥ��Ӧ��ˮ�ⷴӦ���ʴ�Ϊ��AC��
II��A�����������ӳɷ�Ӧ����3-�����飬A����������6��̼ԭ�ӣ���A�Ľṹ��ʽΪ��CH2=C��CH2CH3��CH2CH2CH2CH3��A���廯�ⷢ���ӳɷ�Ӧ����B�����������Ϣ֪��B�Ľṹ��ʽΪ��CH2BrCH��CH2CH3��CH2CH2CH2CH3��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����C��C�Ľṹ��ʽΪ��CH2OHCH��CH2CH3��CH2CH2CH2CH3��D�ǶԶ��ױ���һ��ͬ���칹�壬D�����Ը��������������E�ᣬE�ı����ϴ���2�ֲ�ͬ��ѧ��������ԭ�ӣ���D���ڶ��ױ���E�Ľṹ��ʽΪ��
 ��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ��
��E��C��Ӧ����DEHP��C24H38O4����DEHP�Ľṹ��ʽΪ�� ��
����1��ͨ�����Ϸ���֪��E�Ľṹ��ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��B���������Ƶ�ˮ��Һ����ȡ����Ӧ����ˮ�ⷴӦ������C������B��C�ķ�Ӧ������ȡ����Ӧ��ˮ�ⷴӦ��
�ʴ�Ϊ��ȡ����Ӧ��ˮ�ⷴӦ��
��3��3-�������ijͬ���칹��˴Ź���������ʾֻ��һ���⣬˵����������ֻ��һ�����͵���ԭ�ӣ�������ṹ��ʽΪ����CH3��3CC��CH3��3��������Ϊ��2��2��3��3-�ļ����飬
�ʴ�Ϊ����CH3��3CC��CH3��3��2��2��3��3-�ļ����飻
��4��ͨ�����Ϸ���֪��DEHP�Ľṹ��ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����5��F�Ľṹ��ʽ�з�������������F�DZ�����λ��ȡ���˵��F�к��б�������ȡ��λ��Ϊ�����ϵ���λ����FeCl3��Һ����ɫ��˵��F�к��з��ǻ���F����̼��������Һ��Ӧ��˵��F�����Ȼ�������F�Ľṹ��ʽΪ��
 ��F��̼��������Һ��Ӧ�ķ���ʽΪ��
��F��̼��������Һ��Ӧ�ķ���ʽΪ�� ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ����ݷ�Ӧ�����������ŵı仯ȷ�������Ļ�ѧ��Ӧ��ע���������Ϣ���з�������ѵ���ͬ���칹����жϣ��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ