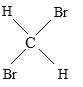
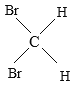
��Ŀ����
����Ŀ��ij��ѧ��ȤС����ʵ���ҴӺ���������ȡ�Ⲣ�Ʊ�KI���塣��ش���������
(1)��ˮ��Һ����ȡ�����ѡ�õ��Լ���____________��(�����)
A���ƾ� B��CCl4 C����ϩ D��ֱ������
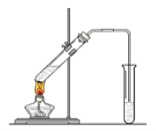
(2)KI������Ʊ���ʵ��װ����ͼ��

ʵ�鲽������
i������0.5mol��L1��KOH��Һ��
i��������ƿ�м���12.7g����I2��250mL 0.5mol��L1��KOH��Һ������������ȫ�ܽ⡣
����ͨ����Һ©����Ӧ�����Һ�еμ��������ᣬ��ַ�Ӧ��HCOOH������ΪCO2������KOH��Һ��pH��9~10����������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ������KI��Ʒ8.3g����ش��������⣺
������0.5mol��L1 KOH��Һʱ�����в���������õ���ҺŨ��ƫ�ߵ���_____(�����)��
A�������Ϸֱ����������ȵ�ֽƬ�����KOH����
B��KOH������Ʒ�л���K2O2
C�������õĹ�������ձ����ܽ�δ����ȴֱ��ת��������ƿ
D��δϴ���ձ���������ֱ��������ƿ�м�ˮ����
E������ʱ���ӿ̶���
�ڲ��袢��I2��KOH��Һ��Ӧ���ɵ���������ͻ�ԭ��������ʵ���֮��Ϊ1��5����д����������Ļ�ѧʽ��____________��
�۲��袣������Һ�еμ���������ʱ�������___________��(�a����b����a��b��)
��ʵ���У�����HCOOH����������ԭ��Ӧ�����ӷ���ʽΪ____________________��
��ʵ����KI�IJ���Ϊ________________%
���𰸡�BD BC KIO3 b 3HCOOH +IO3-===I-+3CO2��+ 3H2O 50
��������
��1����ˮ��Һ����ȡ�⣬��Ϊ��ȡ��������Ҫ���㣺��������ȡ�����ܽ�Ƚϴ���ȡ����ԭ�ܼ������ʾ�����Ӧ����ȡ����ԭ�ܼ������ܡ�
A.�ƾ����ˮ����Ӧ������ˮ���ܣ��ʲ�ѡA��
B.���Ȼ�̼���ˮ����Ӧ����ˮ�����ܣ��ҵ������Ȼ�̼�е��ܽ�ȸ���ѡB��
C.��ϩ���Ժ͵ⷴӦ����������ȡ�����ʲ�ѡC��
D.ֱ�����͵���Ҫ�ɷ��������뻷���������ˮ����Ӧ����ˮ�����ܣ��ҵ������е��ܽ�ȸ���ѡD����С���Ϊ��BD��
��2����A.KOH���������ˮ�ԣ�����������ж�����̼��Ӧ����ֽƬ�ϳ���KOH���壬��ʹ��������KOH����ƫС��������õ���ҺŨ��ƫ�ͣ��ʲ�ѡA��
B.1molK2O2��ˮ��Ӧ����2molKOH��1molK2O2������Ϊ110g����2molKOH������Ϊ112g����KOH������Ʒ�л���K2O2��ʹ������Һ������KOH�����ʵ�����������õ���ҺŨ��ƫ�ߣ���ѡB��
C.��Һδ����ȴ��ע������ƿ����ȴ����Һ�����С������Ũ��ƫ��ѡC��
D.δϴ���ձ���������ֱ��������ƿ�м�ˮ���ݣ��������ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ��ʲ�ѡD��
E.����ʱ���ӿ̶��ߣ�������Һ�����ƫ������������Һ��Ũ��ƫ�ͣ��ʲ�ѡE����С���Ϊ��BC��
��I2��KOH��Һ��Ӧ��I2�ȱ������ֱ���ԭ�������жϻ�ԭ����ΪKI�������ɵ���������ͻ�ԭ��������ʵ���֮��Ϊ1��5�����ݵ�ʧ�����غ㣬���жϳ����������е�Ԫ�ػ��ϼ�Ϊ+5�ۣ����Ƴ���������ΪKIO3����С���Ϊ��KIO3��
�۲��袣������Һ�еμ���������ʱ��ʹ�õ��Ǻ�ѹ��Һ©��������ƽ��ѹǿ���ã����ֻ�����b��������ʹ����˳�����¡���С���Ϊ��b��
������Ϣ��֪���������������ǻ�ԭ����أ�HCOOH������ΪCO2�����ݵ�ʧ�����غ�͵���غ�д��HCOOH����������ԭ��Ӧ�����ӷ���ʽΪ��3HCOOH +IO3-===I-+3CO2��+ 3H2O����С���Ϊ��3HCOOH +IO3-===I-+3CO2��+ 3H2O��
��12.7gI2�����ʵ���Ϊ0.05mol��KOH�����ʵ���Ϊ0.125mol����![]() ��֪��KOH������KIO3��HCOOH��ԭҲ����KI����������0.05molI2������0.1molKI������Ϊ16.6g��������ʵ����KI�IJ���Ϊ
��֪��KOH������KIO3��HCOOH��ԭҲ����KI����������0.05molI2������0.1molKI������Ϊ16.6g��������ʵ����KI�IJ���Ϊ![]() ����С���Ϊ��50%��
����С���Ϊ��50%��

 Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�����Ŀ������ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⡣
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� | �� |
��1��10��Ԫ���У���ѧ��������õ���__________��
��2���٢ڢ��У�����������ˮ���������ǿ����__________��
��3��10��Ԫ��������������ˮ���������ǿ����__________��
��4��Ԫ�آ���ɵĺ��Ǽ��Լ��ķ��ӵĵ���ʽ��__________��
��5�����֢١��ڵ�̼�����εļ�ʵ�鷽��__________��
��6���ٺ͢�����������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ__________��
��7���۵ĵ��������������ﷴӦ�ķ���ʽ��__________��