��Ŀ����
3��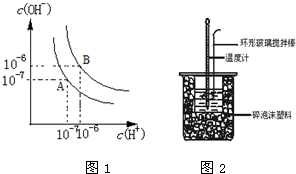 ��Һ������Զ������������Ҫ��Ӱ�죮
��Һ������Զ������������Ҫ��Ӱ�죮��1��ˮ�ĵ��뷽��ʽ��H2O?H++OH-��
��2��ˮ�ĵ���ƽ��������ͼ1��ʾ��ͼ��A���ʾ25��ʱˮ�ĵ���ƽ��״̬����������T��ʱˮ�ĵ���ƽ��״̬ΪB�㣬����A��ˮ�����ӻ���ΪKW1��B��ˮ�����ӻ���ΪKW2����KW1��KW2=1��100��
��3��T��ʱ����pH=5��������pH=8��Ba��OH��2��Һ��ϣ�������T��ĺ��£���ʹ�����Һ��pH=6����������Ba��OH��2��Һ�������Ϊ10��1��
��4��25��ʱ������������������Һ��Ӧ������1molˮʱ�ų�57.3kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽHCl��aq��+$\frac{1}{2}$Ba��OH��2��aq���T$\frac{1}{2}$BaCl2��aq��+H2O��l����H=-57.3 kJ/mol��
��5��25��ʱ����50mL0.5mol/L������50mL0.55mol/LNaOH��Һ��Ӧ�ⶨ�к��ȣ�����˵����ȷ����ab��������ĸ��ţ�
a������ͼ2��ʾװ�òⶨ�к���
b���������β������������ͭ˿���ⶨ�ġ�Hƫ��
c����2����Һ���������Ϊ60mL���ⶨ�ġ�Hƫ��
d�����������Ϊ���ᣬ�ⶨ�ġ�Hƫ�ߣ�
���� ��1��ˮ��������ʣ����ֵ������������ӻ����������ӣ�
��2��ˮ�����ӻ�����Kw=c��H+����c��OH-����
��3��T��ʱ��Kw=10-6.10-6=10-12��������c��H+��=10-5mol/L������������c��OH-��=$\frac{1{0}^{-12}}{1{0}^{-8}}$mol/L=10-4mol/L�������Һ��c��H+��=10-6mol/L����Һ�����ԣ����������������ʵ������ڼ����������������ʵ������ݴ˼���������֮�ȣ�
��4����������кͷ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������д�Ȼ�ѧ����ʽ��
��5��a����װ�÷����к��Ȳⶨ�������ܲⶨ�к��ȣ�
b�������β������������ͭ˿��ͭ���е����ԣ��ᵼ�²�������ɢʧ��
c���к�����������ʵ����أ�
d�����������������ȹ��̣�
��� �⣺��1��ˮ��������ʣ����ֵ������������ӻ����������ӣ����뷽��ʽΪH2O?H++OH-���ʴ�Ϊ��H2O?H++OH-��
��2��ˮ�����ӻ�����Kw=c��H+����c��OH-������KW1��KW2=10-14��10-12=1��100���ʴ�Ϊ��1��100��
��3��T��ʱ��Kw=10-6.10-6=10-12��������c��H+��=10-5mol/L������������c��OH-��=$\frac{1{0}^{-12}}{1{0}^{-8}}$mol/L=10-4mol/L�������Һ��c��H+��=10-6mol/L����Һ�����ԣ����������������ʵ������ڼ����������������ʵ���������������������֮��=10-4mol/L��10-5mol/L=10��1���ʴ�Ϊ��10��1��
��4����������кͷ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������д�Ȼ�ѧ����ʽ�����Ȼ�ѧ����ʽΪHCl��aq��+$\frac{1}{2}$Ba��OH��2��aq���T$\frac{1}{2}$BaCl2��aq��+H2O��l����H=-57.3 kJ/mol��
�ʴ�Ϊ��HCl��aq��+$\frac{1}{2}$Ba��OH��2��aq���T$\frac{1}{2}$BaCl2��aq��+H2O��l����H=-57.3 kJ/mol��
��5��a����װ�÷����к��Ȳⶨ�������ܲⶨ�к��ȣ�����ȷ��
b�������β������������ͭ˿��ͭ���е����ԣ��ᵼ�²�������ɢʧ�����²ⶨ�ġ�Hƫ��
������ȷ��
c���к�����������ʵ����أ����Բⶨ�к��Ȳ��䣬�ʴ���
d�����������������ȹ��̣����Զ��߷�Ӧ�����д���������ղ������������²ⶨ�ġ�Hƫ�ͣ��ʴ���
��ѡab��
���� ���⿼�����ӻ������йؼ��㡢�к��ȵIJⶨ��֪ʶ�㣬Ϊ��Ƶ���㣬ע�����ӻ��������¶��йأ�����Һ����Լ�Ũ���أ�ע���к��Ȳⶨ����������ʵ��ԭ�����״�ѡ���ǣ�5����d����������ΪŨ����ᵼ�²������ƫ�ߣ�

| A�� | 84% | B�� | 60% | C�� | 91% | D�� | 42% |
| A�� | ���ɱ����ǻ����� | B�� | �����Ǽ� | ||
| C�� | K2SO4��K2CO3�����ڼ��� | D�� | �����ǽ��� |
 ��Ӧ2NO2��g��?N2O4��g����H=-57kJ•mol-1�����������¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǣ�������
��Ӧ2NO2��g��?N2O4��g����H=-57kJ•mol-1�����������¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��״̬B��״̬A�������ü��ȵķ��� | |
| B�� | A��C�����������ɫ��A�Cdz | |
| C�� | A��C����ķ�Ӧ���ʣ�A��C | |
| D�� | A��C���������ƽ����Է���������A��C |
| A�� | N2��g��+3H2��g��?2NH3��g�� | B�� | H2��g��+I2��g��?2HI��g�� | ||
| C�� | C��s��+H2O��g��?CO��g��+H2��g�� | D�� | CO2��g��+2NH3��g��?CO��NH2��2��s��+H2O��g�� |
| A�� | �ۢ٢ܢڢ� | B�� | �٢ۢܢڢ� | C�� | �ۢڢ٢ܢ� | D�� | �ݢڢܢ٢� |