��Ŀ����
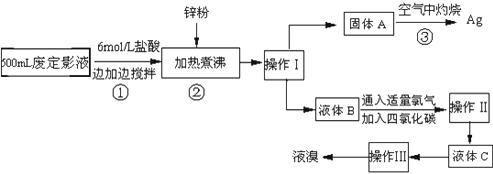
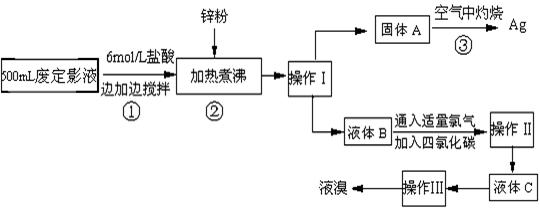
��10�֣������õķ϶�ӰҺ�к���Na����[Ag(S2O3)2]3����Br�������ӡ�ij�о���ѧϰС����ͨ������ʵ�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������塣����֪��4H��+2 [Ag(S2O3)2]3��= Ag2S��+3S��+3SO2��+SO42��+2H2O��
�Ų������������ �� ����������Ҫ����Ҫ���������� �� ��
�Ƽ���п�۵�Ŀ���ǽ������廯���е�����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ ����
��Һ��B�г�����Br��������SO42����������Һ�д���SO42���IJ����� �� ��
�Ȳ����ʵ�����ʱ��Ҳ�����ü�����������ؼӸǺ������ȵķ������Ʋ������ص�Ŀ�Ŀ����� �� ��
��ͨ�������������������У�����ɫ��ѧ��Ҫ�����ڵIJ���Ϊ �� ��
��10�֣�
������(1��) ��Һ©�����ձ�(2��)
��2AgBr + Zn =" 2Ag" + Zn2+ + 2Br�� (2��)
��ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42��(2��)
���ṩ����ʹAg2S��ַ�Ӧ(2��)
���ڿ��������ղ�����SO2�ж�����Ⱦ����(1��)
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 .
.