��Ŀ����
�����õķ϶�ӰҺ�к���Na+��[Ag��S2O3��2]3-��Br-�����ӣ�ij�о���ѧϰС����ͨ������ʵ�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������壮����֪��4H++2[Ag��S2O3��2]3-�TAg2S��+3S��+3SO2��+SO42-+2H2O��
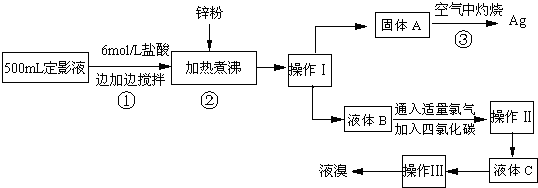
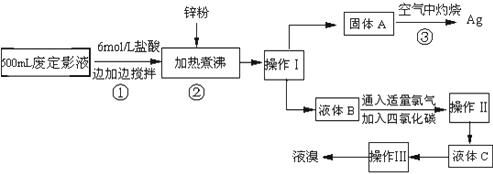
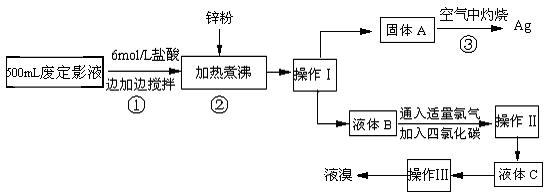
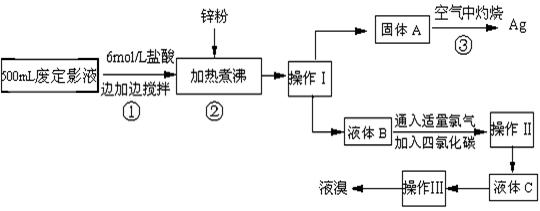
��1�������������
��2������п�۵�Ŀ���ǽ������廯���е�����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ
��3��Һ��B�г�����Br-������SO42-��������Һ�д���SO42-�IJ�����
��4�������ʵ�����ʱ��Ҳ�����ü�����������ؼӸǺ������ȵķ������Ʋ������ص�Ŀ�Ŀ�����
��5��ͨ�������������������У�����ɫ��ѧ��Ҫ�����ڵIJ���Ϊ

��1�������������
����
����
����������Ҫ����Ҫ������������Һ©�����ձ�
��Һ©�����ձ�
����2������п�۵�Ŀ���ǽ������廯���е�����ԭ�������÷�Ӧ�����ӷ�Ӧ����ʽΪ
2AgBr+Zn�T2Ag+Zn2++2Br-
2AgBr+Zn�T2Ag+Zn2++2Br-
����3��Һ��B�г�����Br-������SO42-��������Һ�д���SO42-�IJ�����
ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42-
ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42-
����4�������ʵ�����ʱ��Ҳ�����ü�����������ؼӸǺ������ȵķ������Ʋ������ص�Ŀ�Ŀ�����
�ṩ����ʹAg2S��ַ�Ӧ
�ṩ����ʹAg2S��ַ�Ӧ
����5��ͨ�������������������У�����ɫ��ѧ��Ҫ�����ڵIJ���Ϊ
�ڿ��������ղ�����SO2�ж�����Ⱦ����
�ڿ��������ղ�����SO2�ж�����Ⱦ����
����������1����������Ȼ�̼�з����嵥�ʣ���Ҫͨ�����������ɣ�������ˮ��Һ�����Ȼ�̼��Һ���룬���õ��Ƿ�Һ������ʹ�õ���Һ©�����ձ���
��2��п�Ļ�ԭ�Դ������ģ��������û���Ӧ��
��3�����ݼ�����������ӵķ�����ɣ�
��4������ؿ����ṩ��Ԫ�أ�ʹ�����������ӳ�֣�
��5���������ջ�����ж��Ķ���������Ⱦ������
��2��п�Ļ�ԭ�Դ������ģ��������û���Ӧ��
��3�����ݼ�����������ӵķ�����ɣ�
��4������ؿ����ṩ��Ԫ�أ�ʹ�����������ӳ�֣�
��5���������ջ�����ж��Ķ���������Ⱦ������
����⣺��1�����ڴ�������Ȼ�̼�з����嵥�ʣ���Ҫͨ�����������ɣ�������ˮ��Һ�����Ȼ�̼��Һ���룬���õ��Ƿ�Һ������ʹ�õ���Һ©�����ձ���
�ʴ�Ϊ������Һ©�����ձ���
��2������п���廯����Һ��Ӧ���ɵ���������Ӧ�����ӷ���ʽΪ2AgBr+Zn�T2Ag+Zn2++2Br-���ʴ�Ϊ��2AgBr+Zn�T2Ag+Zn2++2Br-��
��3��Һ��B�г�����Br-������SO42-��������Һ�д���SO42-�ķ���Ϊ��ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42-��
�ʴ�Ϊ��ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42-��
��4����������أ�����������ṩ��Ԫ�أ�����ʹ������ַ�Ӧ���ʴ�Ϊ���ṩ����ʹAg2S��ַ�Ӧ��
��5������A�����������ջ�����ж����ʶ���������ɴ�����Ⱦ���ʴ��ǣ��ڿ��������ղ�����SO2�ж�����Ⱦ������
�ʴ�Ϊ������Һ©�����ձ���
��2������п���廯����Һ��Ӧ���ɵ���������Ӧ�����ӷ���ʽΪ2AgBr+Zn�T2Ag+Zn2++2Br-���ʴ�Ϊ��2AgBr+Zn�T2Ag+Zn2++2Br-��
��3��Һ��B�г�����Br-������SO42-��������Һ�д���SO42-�ķ���Ϊ��ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42-��
�ʴ�Ϊ��ȡ����Һ��B��С�Թ��У��μ�BaCl2��Һ���а�ɫ����˵����SO42-��
��4����������أ�����������ṩ��Ԫ�أ�����ʹ������ַ�Ӧ���ʴ�Ϊ���ṩ����ʹAg2S��ַ�Ӧ��
��5������A�����������ջ�����ж����ʶ���������ɴ�����Ⱦ���ʴ��ǣ��ڿ��������ղ�����SO2�ж�����Ⱦ������
���������⿼����������Ļ��ռ��Ͻ𱣻��������ؼ���Ҫ֪�����˷���������ص㣬�˽⻯ѧ����ʽ��д������Һϡ�͵ķ������õ�����������Ϥ�û���Ӧ���ص㣬�����Ѷ��еȣ�

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
 .
.