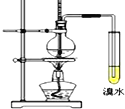
��Ŀ����
����Ŀ���屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�

�� | �� | �屽 | |
�ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
��1���벹��ͼ1��ƿ�з�Ӧ�Ļ�ѧ����ʽ��
��2Fe+3Br2=2FeBr3 ��______________________________��
��2��ͼ1ϴ��ƿ��ʢ�ŵ��Լ�Ϊ__________��ʵ���й۲쵽ϴ��ƿ ��Һ���Ϊ�Ȼ�ɫ�����۲쵽��ƿ�г��ְ�����������Ϊ____________________�γɵġ�
��3���Ƶõ��屽�������в�������ᴿ��

�ٲ��������л�����__________�����������ƣ��д���__________�㡣
�ڲ�������NaOH��Һ��������____________________��
�����л���3�����Ȼ��Ƶ�Ŀ����______________________________��
�ܾ����ϲ�����Ҫ���л���4��һ���ᴿ�����в����б������__________����ѡ���
a���ؽᾧ b������ c������ d����ȡ
��4����ʹ��ͼ2��ʾװ����ȡ�屽����������������ȴˮӦ��__________�ڽ��룬��ѹ��Һ©����ʢ�ŵ��Լ�ӦΪ__________����ѡ���
a���� b��Һ��
���𰸡�  CCl4 HBr������ˮ�����γ����� ��Һ©�� �� ��ȥ�屽���嵥�� ��ȥ�屽�е�ˮ c �� b
CCl4 HBr������ˮ�����γ����� ��Һ©�� �� ��ȥ�屽���嵥�� ��ȥ�屽�е�ˮ c �� b
����������1��ͼ1��ƿ�У�����Һ����FeBr3�������������·���ȡ����Ӧ�����屽����ѧ����ʽΪ�� ��
��
��2��ϴ��ƿ�б�Ȼ�ɫ����ϴ��ƿ������Br2������Һ����лӷ��ԣ���Br2������CCl4������ͼ1ϴ��ƿ�п�ʢװCCl4���ջӷ�����Br2������Һ�巴Ӧ����HBr��HBr��ˮ������ϳʰ�����
�ʴ�Ϊ��CCl4��HBr������ˮ�����γ�������
��3�����屽�к����屽������δ��Ӧ���Br2��FeBr3�������屽ˮϴ��Һ��ֲ㣬FeBr3������ˮ����ˮ�㣬���������л���ͱ����屽һ������л��㣬��Һ���л���1���ټ�NaOH��Һ��ȥBr2���ٴη�Һ��ˮ������Ҫ����NaBr��NaBrO�ȣ��л���2�к��б����屽���ڶ���ˮϴ��ȥ�л����п��ܺ��е�����NaOH����Һ����л���3��������ˮ�Ȼ�����ˮ�����ˣ����л���4�����к��б����屽��
�����������������Ϊ��Һ���屽���ܶȴ���ˮ�����Է�Һ���л����ڷ�Һ©���д����²㡣�ʴ�Ϊ����Һ©�����¡�
�ڸ���������������II��NaOH�������ǣ���ȥ�屽���嵥����
�����л���3�м����Ȼ��Ƶ�Ŀ���ǣ���ȥ�屽�е�ˮ��
���л���4�к��б����屽�����ݱ����屽�ķе㲻ͬ���ɽ���������롣��ѡc��
��4���������е�ˮ�����ǡ��½��ϳ�����Һ��ҩƷ�ļ���˳��Ϊ���ܶȴ�ĵ��뵽�ܶ�С����Һ�У�����ܶȴ��ڱ������Ժ�ѹ��Һ©����ʢ�ŵ��Լ�ΪҺ�壬��ѡb���ʴ�Ϊ���£�b��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�