��Ŀ����
20����ҵ���������β���к��е�������NOx��NO��NO2�Ļ������費��N2O4��������̬���������ཡ�������ϴ����в����1����ҵ�Ͽ��ð������շ�����NOx����Ӧԭ�����£�4xNH3+6NOx$\frac{\underline{\;����\;}}{\;}$��2x+3��N2+6xH2O
ij��ѧ��ȤС��ģ��ô������̵�ʵ��װ���磺
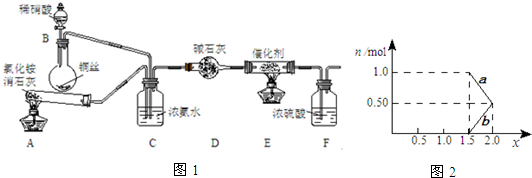
��װ��A�з�����Ӧ�Ļ�ѧ����ʽ2NH4Cl+Ca��OH��2$\frac{\underline{\;��\;}}{\;}$CaCl2+2NH3��+2H2O��
����ͬѧ��Ϊȥ��װ��AҲ���Դﵽʵ��Ŀ�ģ���������Ũ��ˮ���лӷ��ԣ�NOx����װ��C�л����������
��2����ҵ��Ҳ����Na2CO3��Һ���շ�����NOx��
��֪��NO������Na2CO3��Һ��Ӧ��
NO+NO2+Na2CO3=2NaNO2+CO2 ��I��
2NO2+Na2CO3=NaNO2+NaNO3+CO2 ��II��
�ٵ�NOx��Na2CO3��Һ��ȫ����ʱ��x��ֵ��������B������ĸ����
A��1.9B��1.3C��1.6
�ڽ�1mol NOxͨ��Na2CO3��Һ�У�����ȫ����ʱ����Һ�����ɵ�NO3����NO2���������ӵ����ʵ�����x�仯��ϵ��ͼ2��ʾ��ͼ���߶�b��ʾNO3-������xֵ�仯�Ĺ�ϵ������������������Ϊ21.2%�� Na2CO3��Һ���գ�����ҪNa2CO3��Һ����250g��
����������Na2CO3��Һ��ȫ����NOx��ÿ����22.4L����״����CO2��ȫ���ݳ���ʱ������Һ����������40g����NOx�е�xֵΪ$\frac{7}{4}$��
���� ��1�����Ȼ�粒�����������ƹ����ڼ��ȵ������·�����Ӧ���ɰ�����
��Ũ��ˮ���лӷ��ԣ����Բ���������
��2���ٵ�NOx��Na2CO3��Һ��ȫ����ʱ����n��NO2����n��NO�����ݴ����ش�
�����ü������غ㷨��������⼴�ɣ�
�����ò���������NO��NO2�����ʵ������ٸ���ƽ��Ħ������������xֵ��
��� �⣺��1�����Ȼ�粒�����������ƹ����ڼ��ȵ������·�����Ӧ���ɰ���������װ��A�ķ�Ӧ�ǣ�2NH4Cl+Ca��OH��2$\frac{\underline{\;��\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;��\;}}{\;}$CaCl2+2NH3��+2H2O��
��Cװ����Ũ��ˮ���лӷ��ԣ����Բ���������ȥ��װ��AҲ���Դﵽʵ��Ŀ�ģ�
�ʴ�Ϊ��Ũ��ˮ���лӷ��ԣ�NOx����װ��C�л����������
��2�����ɷ���ʽ��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С��x��СֵΪ$\frac{2+1}{2}$=1.5����Ϊ����NO������x���ֵ��2����x��ȡֵ��ΧΪ1.5��x��2������x��ֵ��������1.3��
�ʴ�Ϊ��B��
���ü�������x=1.5����ӦΪNO��NO2�������ʵ�����Ϊ1��1������ʽ��Ӧ��û��NO3-����aӦ�ñ�ʾNO2-��������b��ʾNO3-��
���غ㷨����Ӧ���ɵ�NaNO3��NaNO2�е�Ԫ������Ԫ��֮��Ϊ1��1������1mol NOx����ȫ����������̼����0.5mol������Ϊ53g�������̼������Һ������Ϊ250g��
�ʴ�Ϊ��NO3-��250��
������NO2�ʹ��Ӧ����CO2Ϊamol��
��NO��NO2�봿�Ӧ������CO2Ϊbmol��
2NO2+Na2CO3=NaNO2+NaNO3+CO2 ��������
1mol��m=48g
amol 48ag
NO+NO2+Na2CO3=2NaNO2+CO2 ��������
1mol��m=32g
bmol 32bg
a+b=1
48a+32b=40
���
a=0.5mol
b=0.5mol
n��NO2��=0.5mol��2+0.25mol=1.5mol
n��NO��=0.5mol
x=$\frac{0.5mol��1+1.5mol��2}{0.5mol+1.5mol}$=$\frac{7}{4}$��
�ʴ�Ϊ��$\frac{7}{4}$��
���� �����Ե�������Ϊ���忼�������ʼ�ķ�Ӧ����ȷ���ʵ������ǽⱾ��ؼ����ѵ��ǣ�2����ļ��㣬Ҫ��Ϸ���ʽ�и���������֮��Ĺ�ϵʽ��𣬻���������Ϣ����xֵ���Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��ѧ����������ʶ���� | |
| B�� | ������ˮӦ��������ɫ�Լ�ƿ�У����������� | |
| C�� | �ڻ�ѧ��Ӧ�У��μӷ�Ӧ�ĸ����ʵ������ȵ��������ʵ���֮�� | |
| D�� | Ӣ����ѧ�Ҳ�������ԭ��ѧ˵��Ϊ������ѧ�ķ�չ�춨�˼�ʵ�Ļ��� |
| A�� | 1mol${\;}_{8}^{16}$OD-���е����ӡ���������Ϊ9NA | |
| B�� | 3.6gʯī��C60�Ļ�����У����е�̼ԭ����Ϊ0.3NA | |
| C�� | ����4.6g��Ԫ�صĹ������ƺ������ƵĻ�����У�������������Ϊ0.3NA | |
| D�� | ��״���£�4.48L HF���еķ�����Ϊ0.2NA |
| ����Ļ�ѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | 1.8��l0-5 | 4.9��l0-10 | K1=4.3��l0-7 K2=5.6��l0-11 |
| A�� | 2CN-+H2O+CO2�T2HCN+CO32- | |
| B�� | ��ͬ�¶�ʱ�������ʵ���Ũ�ȵĸ���ҺpH��ϵ��pH��NaCN����pH��Na2CO3����pH��CH3COONa�� | |
| C�� | ����������μ�ˮ����Һ�ĵ����ԡ�����ĵ���̶ȡ�pH����������С | |
| D�� | 25��ʱ��������������ʵ���Ũ�ȵ�NaCN��Һ������������С��CH3COONa��Һ������������ |
| A�� | ijԪ��ԭ�ӵ�ԭ�Ӱ뾶��111pm | |
| B�� | ij����������ܶ�Ϊ1.8g•cm-3 | |
| C�� | ij����ʯ��ˮ��Ũ����2.0mol•L-1 | |
| D�� | �ù㷺pH��ֽ���ij��Һ��pHΪ6.3 |
| A�� | 1��1��1 | B�� | 3��2��1 | C�� | 6��3��2 | D�� | 1��2��3 |