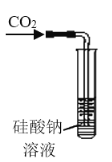
��Ŀ����
����Ŀ����̼�����׳ƹ���˫��ˮ��������Ӧ����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������У������Ʊ�ԭ����·�����£�
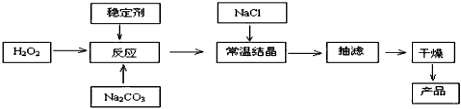
��֪��2Na2CO3 + 3H2O2 =2Na2CO3��3H2O2 ��H<0
��ش��������⣺
��1���������ʿ�ʹ��̼���ƽϿ�ʧЧ����_________________��
a��MnO2 b��H2S c��ϡ���� d��NaHCO3
��2������NaCl��������_________________��
��3����ҵ�����к���Fe3+�����ʣ������ȶ�������������Fe3+�����ȶ�������Fe3+�Է�Ӧ�IJ���Ӱ����_________________��
��4����Ӧ������¶ȿ�����15�桫20�棬�¶�ƫ��ʱ��ɲ��ʵͿ�����_________________��
��5��������������©��һ����������ò�Ʒ����ƫ�ͣ��ò�������������_________________�����иò����ķ����ǣ�_________________��
��6��Ϊ�ⶨ��Ʒ�Ĵ��ȡ�
ȷ��ȡag��Ʒ���250mL��Һ����ȡ25.00mL����ƿ�У�����ϡ�����ữ��������ˮϡ�ͳ�100mL���������������ø�����ر���Һ�ζ�����������MnO4-�Ļ�ԭ������Mn2+����cmol/L KMnO4����ҺVmL�ζ�����Һ���ζ������յ��������________________��
�ظ��ζ����Σ�ƽ������cmol/L KMnO4����ҺVmL�����Ʒ�й�̼���Ƶ���������Ϊ______________������cmol/L KMnO4����Һʱ����Һʱ������Һ�彦�������Ʒ�Ĵ��Ƚ�_________�����С�䣩��
���𰸡�abcʹ2Na2CO3��3H2O2��ˮ�е��ܽ⽵�ͣ��������ྦྷ���˫��ˮ�ķֽ��¶ȸ�ʱ˫��ˮ�ֽ⾧���ϴ�������ڹ������ϵij���������ˮ���պ���û���������ã���ˮ��Ȼ���������ظ����������������Ը��������Һ�������һ��ʱ����Һ����ɫ��Ϊdz��ɫ����30s���ظ�ԭɫ![]() ���
���
��������
(1)���������ܹ����������ٽ�����˫��ˮ�ֽ⣻������л�ԭ�ԣ��ܹ������˫��ˮ��Ӧ��ϡ�����ܹ����̼���Ʒ�Ӧ��̼�����Ʋ������˫��ˮ��Ӧ��
(2)���ݱ�����Һ�е��ܽ�ƽ����з�����
��3���������ܹ����������ٽ�˫��ˮ�ķֽ⣻
��4��˫��ˮ���ȶ����¶ȸ������ֽ⣻�����¶ȵ��ˣ���Ӧ����̫����
��5�����������л���Ҫϴ�Ӳ�����
��6���ζ������յ�������ǣ����Ը��������Һ�������һ��ʱ����Һ����ɫ��Ϊdz��ɫ����30s���ظ�ԭɫ��
���ݹ�̼������Ʒ��KMnO4 �Ĺ�ϵ����μӷ�Ӧ�Ĺ�̼���Ƶ������������������Ʒ�еĺ�����
��Һʱ������Һ�彦����ʹ��ҺŨ��ƫС�����ĵı�Һ���ƫ��
(1)a�����������ܹ����������ٽ�����˫��ˮ�ֽ⣬ʹ����˫��ˮʧЧ����A��ȷ��
b��������л�ԭ�ԣ��ܹ������˫��ˮ��Ӧ��ʹ����˫��ˮʧЧ����B��ȷ��
c��ϡ�����ܹ����̼���Ʒ�Ӧ��ʹ����˫��ˮʧЧ����C��ȷ��
d��̼�����������˫��ˮ����Ӧ������ʹ����˫��ˮʧЧ����D����
������ȷ����abc
(2)�������Ȼ�����������Һ��������Ũ���������˹���˫��ˮ���ܽ�ȣ����������ྦྷ�塣
��3���������ܹ����������ٽ�˫��ˮ�ķֽ⣻
��4������˫��ˮ���ȶ����¶ȸ������ֽ⣻�����¶ȵ��ˣ���Ӧ����̫����
��5��������������Ҫϴ�Ӳ����������������Ϊ�������ڹ������ϵij���������ˮ���պ���û���������ã���ˮ��Ȼ���������ظ������������Σ�
��6���ζ������յ�������ǣ����Ը��������Һ�������һ��ʱ����Һ����ɫ��Ϊdz��ɫ����30s���ظ�ԭɫ��
��μӷ�Ӧ��2Na2CO3��3H2O2Ϊnmol��
���ݹ�ϵʽ��
6KMnO4������������������������5��2Na2CO3��3H2O2��
6mol 5mol
��c mol��L-1��v mL��10-3 L��mL��1�� n
���� n ��2Na2CO3��3H2O2��=cV5/6��10-3mol
��m ��2Na2CO3��3H2O2��=cV5/6��10-3mol��314g��mol��1
��w��2Na2CO3��3H2O2��=![]() ��
��
����cmol/L KMnO4����Һʱ����Һʱ������Һ�彦����ʹ��ҺŨ��ƫС�����ĵı�Һ���ƫ�����Ʒ�Ĵ��Ƚ����

����Ŀ��a��b��c��d�Ƕ�����Ԫ�أ������ڱ��е����λ����ͼ��ʾ��dԪ��ԭ�Ӻ���M���������K���������2��������˵���У�������ǣ� ��
a | b | c |
d |
A. �����£�a���ʿ���d����������û���Ӧ
B. b����̬�⻯���������ۺ����ᷴӦ
C. a��b��c ����������ϼ۵���������������
D. d������������������IJ���