��Ŀ����
ij������ˮ��Һ�����ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����NH4+��Cl����CO32����SO42�����ֱַ�ȡ100 mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02 g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65 g���塣
(1)һ�������ڵ�������________(�����ӷ��ţ���ͬ)��
(2)�ɢٿ�֪��������Ϊ________��Ũ��________���ɢڿ�֪��������Ϊ________��Ũ��________��
�ɢۿ�֪��������Ϊ________��Ũ��________��
(3)K���Ƿ���ڣ�________(��ǡ���)��������___________________________
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02 g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65 g���塣
(1)һ�������ڵ�������________(�����ӷ��ţ���ͬ)��
(2)�ɢٿ�֪��������Ϊ________��Ũ��________���ɢڿ�֪��������Ϊ________��Ũ��________��
�ɢۿ�֪��������Ϊ________��Ũ��________��
(3)K���Ƿ���ڣ�________(��ǡ���)��������___________________________
��(1)Fe3����Mg2����Ba2����CO32����(2)NH4+
0��2 mol/L ��Al3����0.2 mol/L ��SO42����0.5 mol/L
(3)�ǡ����ݵ���غ㣬���������������С�������Ӹ��������������һ����K�����ڡ�
0��2 mol/L ��Al3����0.2 mol/L ��SO42����0.5 mol/L
(3)�ǡ����ݵ���غ㣬���������������С�������Ӹ��������������һ����K�����ڡ�
���ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02 mol���������ɣ�˵������Һ�в���Fe3����Mg2��������NH4+�������ʵ�����0.02 mol���������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02 g���壬˵������Һ�к���Al3���������ʵ�����0.02 mol���۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65 g���壬˵������Һ�к�����������ӣ����Բ��������ӣ���������ӵ����ʵ�����0.05 mol�����ݵ���غ㣬���������������С�������Ӹ��������������һ����K�����ڡ�

��ϰ��ϵ�д�
�����Ŀ
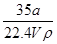 ��100%
��100%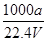

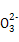 ����NA
����NA