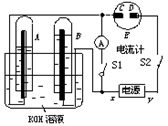
��Ŀ����
��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺��1�������Դ������������xΪ______��
��2������ֽ��C�˸������۲쵽��������______��?
��3��д���缫��Ӧʽ��B�缫______��?
��4�������һ��ʱ���A��B�о��������Χ�缫����ʱ�жϿ���S2�պϿ���S1��������Ƶ�ָ���Ƿ���ƫת______���ƫת����ƫת������?
��5����������ָ��ƫת��д���йصĵ缫��Ӧ����ָ�롰��ƫת�������ⲻ�ػش𣩣�______����������ָ�벻ƫת����˵�����ɣ���ָ�롰ƫת�������ⲻ�ػش�______��
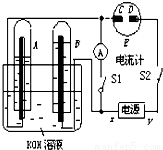
���𰸡���������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2����ΪA�Թ������������B�Թ��е�2��������A��ΪH2��B��ΪO2����AΪ������BΪ����������xΪ������yΪ������C���Դ����������Ϊ�������缫��Ӧ����ʽΪ2I--2e-=I2��B�缫����������Һ�е�OH-�ŵ磬�缫��Ӧʽ��4OH--4e-=2H2O+O2��������ж�S2���պ�S1�������A�е�H2��B�е�O2��KOH��Һ�γ�H2-O2ȼ�ϵ�أ�����һ���Ǹ���������һ����������
����⣺��1�����Ե缫���KOH��Һ��ʵ�ʾ��ǵ��ˮ�����ò���ΪH2��O2����ΪA�Թ������������B�Թ��е�2��������A��ΪH2��B��ΪO2����AΪ������BΪ����������xΪ������yΪ�������ʴ�Ϊ������
��2��C���Դ����������Ϊ�������缫��Ӧ����ʽΪ2I--2e-=I2��������������ɫ��������ֽ�������ʴ�Ϊ����ֽ������
��3��B�缫����������Һ�е�OH-�ŵ磬�缫��Ӧʽ��4OH--4e-=2H2O+O2�����ʴ�Ϊ��4OH--4e=2H2O+O2����
��4������ж�S2���պ�S1�������A�е�H2��B�е�O2��KOH��Һ�γ�H2-O2ȼ�ϵ�أ��ѻ�ѧ�ܱ�Ϊ���ܣ����ָ��ƫת���ʴ�Ϊ��ƫת��
��5����ԭ����и���ʧȥ���ӣ������õ����ӣ���������һ���Ǹ���������һ�����������缫��Ӧʽ�ֱ��ǣ�A�� 2H2+4OH--4e-=4H2O��B�� 2H2O+O2+4e-=4OH-��
�ʴ�Ϊ��2H2+4OH--4e=4H2O��2H2O+O2+4e=4OH-��
�����������ۺϿ���ԭ��غ͵���֪ʶ���Ǹ߿��еij������ͺ���Ҫ�Ŀ���֮һ�������е��Ѷȵ����⣮�����ۺ���ǿ���������У������߿�������������ѧ���������⡢��������������Ҳ����������ѧ������˼ά�����ͷ�ɢ˼ά���������ѧ��ѧϰЧ�ʣ�
����⣺��1�����Ե缫���KOH��Һ��ʵ�ʾ��ǵ��ˮ�����ò���ΪH2��O2����ΪA�Թ������������B�Թ��е�2��������A��ΪH2��B��ΪO2����AΪ������BΪ����������xΪ������yΪ�������ʴ�Ϊ������
��2��C���Դ����������Ϊ�������缫��Ӧ����ʽΪ2I--2e-=I2��������������ɫ��������ֽ�������ʴ�Ϊ����ֽ������
��3��B�缫����������Һ�е�OH-�ŵ磬�缫��Ӧʽ��4OH--4e-=2H2O+O2�����ʴ�Ϊ��4OH--4e=2H2O+O2����
��4������ж�S2���պ�S1�������A�е�H2��B�е�O2��KOH��Һ�γ�H2-O2ȼ�ϵ�أ��ѻ�ѧ�ܱ�Ϊ���ܣ����ָ��ƫת���ʴ�Ϊ��ƫת��
��5����ԭ����и���ʧȥ���ӣ������õ����ӣ���������һ���Ǹ���������һ�����������缫��Ӧʽ�ֱ��ǣ�A�� 2H2+4OH--4e-=4H2O��B�� 2H2O+O2+4e-=4OH-��
�ʴ�Ϊ��2H2+4OH--4e=4H2O��2H2O+O2+4e=4OH-��
�����������ۺϿ���ԭ��غ͵���֪ʶ���Ǹ߿��еij������ͺ���Ҫ�Ŀ���֮һ�������е��Ѷȵ����⣮�����ۺ���ǿ���������У������߿�������������ѧ���������⡢��������������Ҳ����������ѧ������˼ά�����ͷ�ɢ˼ά���������ѧ��ѧϰЧ�ʣ�

��ϰ��ϵ�д�
�����Ŀ
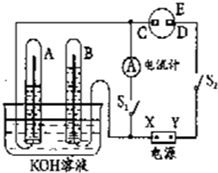 ����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺
����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺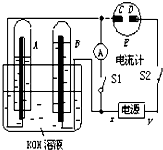 ��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺
��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺ ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��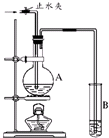 ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��