��Ŀ����
��13�֣������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ��壨Na2S2O3��5H2O����
I��[��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��.[�Ʊ���Ʒ]
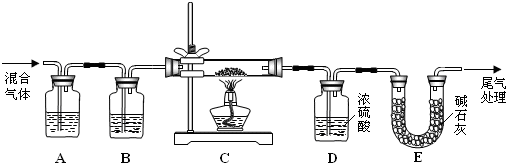
ʵ��װ����ͼ��ʾ��ʡ�Լг�װ�ã�
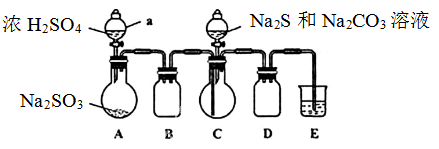
ʵ�鲽�裺
��1�����װ�������ԣ���ͼʾ�����Լ�������a��������____��E�е��Լ���___��ѡ��������ĸ��ţ���
A��ϡH2SO4 B��NaOH��Һ C������NaHSO3��Һ
��2������C����ƿ����Na2S��Na2CO3�����Һ������A����ƿ�μ�ŨH2SO4��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ��____����д�������ƣ����ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��[̽���뷴˼]
��1��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫���������������������Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�_____��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2��Ϊ����װ��C�����ɵ�Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ԭ��ʵ�鲽�裨2�������˸Ľ����Ľ���IJ�����_______��
��3��Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��_____�����ᴿ��
I��[��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��.[�Ʊ���Ʒ]
ʵ��װ����ͼ��ʾ��ʡ�Լг�װ�ã�

ʵ�鲽�裺
��1�����װ�������ԣ���ͼʾ�����Լ�������a��������____��E�е��Լ���___��ѡ��������ĸ��ţ���
A��ϡH2SO4 B��NaOH��Һ C������NaHSO3��Һ
��2������C����ƿ����Na2S��Na2CO3�����Һ������A����ƿ�μ�ŨH2SO4��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ��____����д�������ƣ����ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��[̽���뷴˼]
��1��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫���������������������Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�_____��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2��Ϊ����װ��C�����ɵ�Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ԭ��ʵ�鲽�裨2�������˸Ľ����Ľ���IJ�����_______��
��3��Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��_____�����ᴿ��
��1����Һ©����B ��3������
�� ��1�����ˣ�������ˮϴ�ӳ�����������м�������ϡHCl��
��2������A����ƿ�μ�Ũ���ᣬ���������彫װ���п����ž�������C����ƿ����Na2S��Na2CO3�����Һ����3���ؽᾧ
�� ��1�����ˣ�������ˮϴ�ӳ�����������м�������ϡHCl��
��2������A����ƿ�μ�Ũ���ᣬ���������彫װ���п����ž�������C����ƿ����Na2S��Na2CO3�����Һ����3���ؽᾧ
�����������.[�Ʊ���Ʒ]
��1�����������Ĺ����ص��֪������a�������Ƿ�Һ©��������װ�ÿ�֪��Aװ�����Ʊ�SO2�ģ�Cװ�����Ʊ���Na2S2O3��BDװ���Ƿ������ģ�����SO2�ж�����Ҫβ�����������Eװ��������SO2�ġ�����SO2���������������������������Һ���գ���E�е��Լ�������������Һ����ѡB��
��3������Na2S2O3��5H2O����ɫ�����壬������ˮ�����Ҫ����Һ�еõ���������ƾ��壬������Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��[̽���뷴˼]
��1��Na2S2O3��5H2O��ϡ��Һ��BaCl2��Һ����������ɣ���ʵ��������а�ɫ�������ɣ����Ҫ��һ����֤����������ɫ�����еμ�ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2���������������ױ��������������Σ���װ���к��п����������������������Σ�����Ϊ����װ��C�����ɵ�Na2SO4�������Ľ���Ĵ�ʩ������A����ƿ�μ�Ũ���ᣬ���������彫װ���п����ž�������C����ƿ����Na2S��Na2CO3�����Һ��
��3������Na2S2O3��5H2O���ܽ�����¶�������������������ò�Ʒͨ���ؽᾧ�����ᴿ��

��ϰ��ϵ�д�
�����Ŀ

 NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0