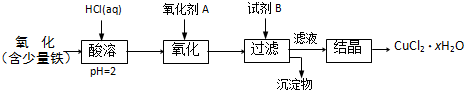
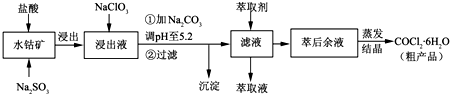
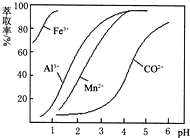
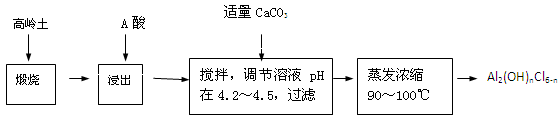
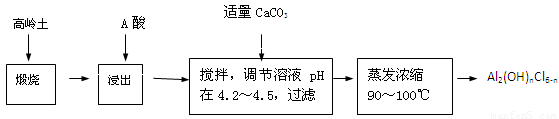
��Ŀ����
��12�֣�
��֪pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu��������n���Լ��缫�ϲ�������������V mL ��״�������ⶨCu�����ԭ���������������£�
�ش��������⣺
��1������CuO�������� ��
��2������������õIJ���������ͼ��ʾ����A��ֱ����Դ�� �����������������
��3����ʼ����U�ι��п��Թ۲쵽�������У�
������ ��������������������������
�������ӷ���ʽ���ܷ�Ӧ��Ϊ
�������������������������������� ��
��4������ʵ������б�Ҫ���� ����д��ĸ����
��A���������ǰ�ĵ缫��������
��B�����缫�ں�ɳ���ǰ������������ˮ��ϴ��
��C�����µ���缫��������ͭ������ϴ��������
��D�������ɳ��صIJ����б��밴����ɡ��������ٺ�ɡ��ٳ��������У�
��E�����п������ڵ�����£���ɵ缫�����õ��º�ɵķ�����
��5��ͭ�����ԭ������Ϊ ���ô���m��V�ļ���ʽ��ʾ����
����11�֣���4��3�֣� ������2��
��1��ͨ������H+��������Һ��pHʹ֮���ߣ�ʹFe3+���ˮ���γ�Fe(OH)3��������ȥ
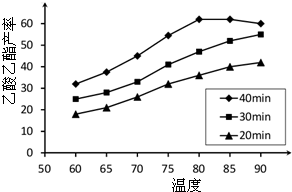
��2������
��3��ʯī���������ݳ�����Һ��ɫ��dz
2Cu2++2H2O==2Cu��+O2��+4H+
��4��A��B��D��E��
��5��11200m/ V
����:

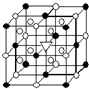 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������