��Ŀ����
��2012?���ݶ�ģ����ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH��l��+C2H5OH��l��
CH3COOC2H5��l��+H2O��l����H=-8.62kJ?mol-1
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78���77�森������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��
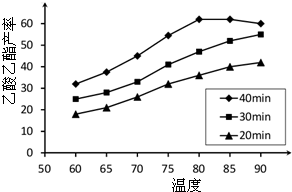
��1�����о�С���ʵ��Ŀ����
��2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����
��3����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������
��4��ij�¶��£���0.10mol CH3COOH����ˮ���1L��Һ��
��ʵ�����ѵ���Ĵ������ռԭ�д������������1.3%������¶���CH3COOH�ĵ���ƽ�ⳣ��K=
�������Һ���ټ���
CH3COOH��l��+C2H5OH��l��
| ŨH2SO4�� |
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118�桢78���77�森������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��

��1�����о�С���ʵ��Ŀ����
̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ��
̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ��
����2��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����
��
��
���ߣ��С�ڡ��������ڡ����ڡ�������3����ͼ��ʾ����Ӧʱ��Ϊ40min���¶ȳ���80��ʱ���������������½���ԭ�������
��Ӧ�����Ѵ�ƽ��״̬���¶�����ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½�
��Ӧ�����Ѵ�ƽ��״̬���¶�����ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½�
��д������������4��ij�¶��£���0.10mol CH3COOH����ˮ���1L��Һ��
��ʵ�����ѵ���Ĵ������ռԭ�д������������1.3%������¶���CH3COOH�ĵ���ƽ�ⳣ��K=
1.7��10-5
1.7��10-5
����ˮ�ĵ�����Բ��ƣ��������Դ������Ũ�ȵ�Ӱ����Բ��ƣ��������Һ���ټ���
1.7��10-2
1.7��10-2
mol CH3COONa��ʹ��Һ��pHԼΪ4������Һ����仯���Բ��ƣ���������1������̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ�죻
��2�����ݲ�����ʱ��ı�ֵ�жϣ�
��3���Ҵ���������лӷ��ԣ�����������ˮ�ⷴӦ�����ȷ�Ӧ�������¶ȶԻ�ѧƽ���Ӱ�������
��4����K=
��
�ڸ��ݻ�����ҺpHֵ�ļ��㷽�����м��㣮
��2�����ݲ�����ʱ��ı�ֵ�жϣ�
��3���Ҵ���������лӷ��ԣ�����������ˮ�ⷴӦ�����ȷ�Ӧ�������¶ȶԻ�ѧƽ���Ӱ�������
��4����K=
| c(H+)��c(CH3COO-) |
| c(CH3COOH) |
�ڸ��ݻ�����ҺpHֵ�ļ��㷽�����м��㣮
����⣺��1������ͼ��֪����ʵ���ʵ��Ŀ���ǣ�̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ�죬
�ʴ�Ϊ��̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ�죻
��2���������������IJ��ʺ�ʱ��ı�ֵ֪��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����С�ں��ߣ��ʴ�Ϊ��С�ڣ�
��3���Ҵ���������лӷ��ԣ�����������ˮ�ⷴӦ�����ȷ�Ӧ������Ӧ�ﵽƽ��״̬ʱ���������¶ȣ��ٽ���������ʱ�䣬ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½���
�ʴ�Ϊ����Ӧ�����Ѵ�ƽ��״̬���¶�����ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½���
��4����K=
=
=1.7��10-5���ʴ�Ϊ��1.7��10-5��
�ڻ�����Һ��pH=pKa-lg
=-lg1.7��10-5-lg
=4��
����c��CH3COONa��=1.7��10-2mol/L����n��CH3COONa��=1.7��10-2mol/L��1L=1.7��10-2mol��
�ʴ�Ϊ��1.7��10-2��
�ʴ�Ϊ��̽����Ӧ�¶ȡ���Ӧʱ��������������ʵ�Ӱ�죻
��2���������������IJ��ʺ�ʱ��ı�ֵ֪��60���·�Ӧ40min��70���·�Ӧ20min��ȣ�ǰ�ߵ�ƽ����Ӧ����С�ں��ߣ��ʴ�Ϊ��С�ڣ�
��3���Ҵ���������лӷ��ԣ�����������ˮ�ⷴӦ�����ȷ�Ӧ������Ӧ�ﵽƽ��״̬ʱ���������¶ȣ��ٽ���������ʱ�䣬ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½���
�ʴ�Ϊ����Ӧ�����Ѵ�ƽ��״̬���¶�����ƽ�����淴Ӧ�����ƶ����¶ȹ��ߣ��Ҵ�����������ӷ�ʹ��Ӧ���������½���
��4����K=
| c(H+)��c(CH3COO-) |
| c(CH3COOH) |
| (0.1mol/L��1.3%)2 |
| 0.1mol/L��(1-1.3%) |
�ڻ�����Һ��pH=pKa-lg
| c(CH3COOH) |
| c(CH3COONa) |
| 0.1 |
| c(CH3COONa) |
����c��CH3COONa��=1.7��10-2mol/L����n��CH3COONa��=1.7��10-2mol/L��1L=1.7��10-2mol��
�ʴ�Ϊ��1.7��10-2��
���������⿼����̽���¶ȶԻ�ѧƽ���Ӱ�졢������ʵĵ��룬�ѵ��Ǽ�������Ƶ����ʵ�������ȷ������Һ��pH���㷽���ǽⱾ��ؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ