��Ŀ����
��ˮ��Ӧ��ǰ�������Ļ���ԭ����Դ,�Ӻ�ˮ�п���ȡ���ֻ���ԭ��.��ͼ�ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ����֪����XΪ��ⱥ��ʳ��ˮ���ã�ĸҺ��±����Ҫ����Ca2+��Mg2+,Cl-,SO42-��Br-������)��ش�
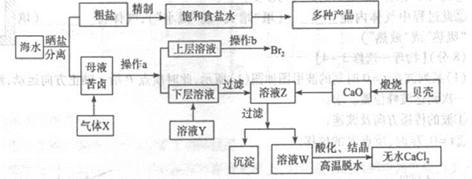
��1���ڴ����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱ���õ��Լ�Ϊ:
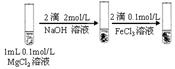
��2������X�Ļ�ѧʽΪ______������a���õ���Ҫ������______��
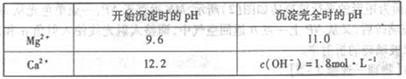
��3��������ҺY��Ŀ����______����CaO������ҺZ��pH,���Գ�ȥMg2+�õ���ҺW���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ��______���ữ��ҺWʱ��ʹ�õ��Լ�Ϊ______
��4��������X����ͨ������װ������֤����X�����ʣ�
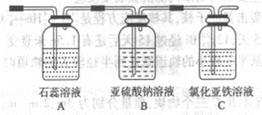
��ͨ������X��A�г��ֵ�������____________
��Cװ���з�����Ӧ�����ӷ���ʽΪ____________��
��������С��ͬѧ���һ��ʵ�飬֤��ϴ��ƿB�е�Na2SO3�ѱ�����(����ʵ�鲽�裩
________________________________________________________________________

��1���ڴ����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱ���õ��Լ�Ϊ:
| A������ | B���Ȼ�����Һ | C������������Һ | D��̼������Һ��������Լ���˳����(����)______ |
��3��������ҺY��Ŀ����______����CaO������ҺZ��pH,���Գ�ȥMg2+�õ���ҺW���ɱ������ݿ�֪�������Ͽ�ѡ��pH���Χ��______���ữ��ҺWʱ��ʹ�õ��Լ�Ϊ______

��4��������X����ͨ������װ������֤����X�����ʣ�

��ͨ������X��A�г��ֵ�������____________
��Cװ���з�����Ӧ�����ӷ���ʽΪ____________��
��������С��ͬѧ���һ��ʵ�飬֤��ϴ��ƿB�е�Na2SO3�ѱ�����(����ʵ�鲽�裩
________________________________________________________________________
��16�֣�
��1��CBDA��BCDA(2��)
��2��Cl2(1��)����Һ©��(1��)
��3����ȥSO42-,��ֹ����CaSO4�ij���(2��)��11.0�QpH�Q12.2(2��)������(2��)
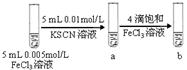
��4������Һ����ɫ�ȱ�죬����Ϊ��ɫ(2��)����2Fe2++Cl2=2Fe3++2Cl-(2��)��
��ȡ������Һ�ڽྻ���Թ��У������еμ�ϡ���������ٲ������壬�������еμ��Ȼ�����Һ����������ɫ��������֤�����������ѱ�������(2��)
��1��CBDA��BCDA(2��)
��2��Cl2(1��)����Һ©��(1��)
��3����ȥSO42-,��ֹ����CaSO4�ij���(2��)��11.0�QpH�Q12.2(2��)������(2��)
��4������Һ����ɫ�ȱ�죬����Ϊ��ɫ(2��)����2Fe2++Cl2=2Fe3++2Cl-(2��)��
��ȡ������Һ�ڽྻ���Թ��У������еμ�ϡ���������ٲ������壬�������еμ��Ȼ�����Һ����������ɫ��������֤�����������ѱ�������(2��)
���������
��1��̼������Һ��ȥ�Ȼ�����Һ���������Ba2+������̼�������Ȼ���������룻�������ڳ�ȥ�������������ơ�̼����������������CBDA��BCDA���ɡ�
��2������Cl2�Ѻ�ˮ��Br-����ΪBr2����ˮ�з����Br2������ȡ�ķ�����ʹ�÷�Һ©����
��3��Ϊ���ƵĽϴ��ĵIJ�Ʒ���˲�Ӧ�ó�ȥSO2-4,��ֹ����CaSO4�ij�����11.0�QpH�Q12.2�ķ�Χ��Mg2+������ȫ����Ca2+�����������Һ����Cl-��������������µ����ʡ�
��4����Cl2+H2O
 HCl+HClO��HClʹ��Һ����ɫ�ȱ�죬HClOʹ��Һ����Ϊ��ɫ�����������Ȼ�������Ӧ��2Fe2++Cl2=2Fe3++2Cl-
HCl+HClO��HClʹ��Һ����ɫ�ȱ�죬HClOʹ��Һ����Ϊ��ɫ�����������Ȼ�������Ӧ��2Fe2++Cl2=2Fe3++2Cl-��5��Na2SO3������������Һ����SO42-�������еμ�ϡ���������ٲ������壬�������еμ��Ȼ�����Һ����������ɫ��������֤�����������ѱ�������

��ϰ��ϵ�д�
�����Ŀ


 8Na2CrO4+2Fe2O3+8CO2
8Na2CrO4+2Fe2O3+8CO2