��Ŀ����
��10�֣���һ���¶��£�������Һ���ڵ���ƽ�⣺
CH3COOH  CH3COO- + H+
CH3COO- + H+
(1��ij�¶�ʱ��0.1mol/L�Ĵ�����Һ�е�c(H+) ��0.01mol/L c(H+)�ı�ֵ _________________������ڡ�����С�ڡ����ڡ���10
��2����֪��25��ʱ���õ���ƽ���ƽ�ⳣ��Ϊ1.75��10-5
������¶�ʱ��amol/L�Ĵ�����Һ��c1(H+)=________________mol/L (�ú�a�Ĵ���ʽ��ʾ)��[��ʾ����ʱa�Ƚ�С�����м��㣬ƽ��ʱc(CH3COOH)���ó�ʼŨ�ȴ��棬ˮ�������c(H+) ��c(OH-)���Բ��ƣ���ͬ]
�������¶�ʱ�����Һ�м���һ������CH3COONH4(������Һ�������)��ʹ��Һ��c(CH3COO-)Ϊbmol/L�����ʱc2(H+)=________________mol/L���ú�a��b�Ĵ���ʽ��ʾ��.
��c1(H+)__________ c2(H+)������ڡ�����С�ڡ����ڡ���
[��Դ:]
��10�֣�(1��<
��2�� �� 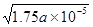 ��(1.75a��10-5)/b ��>
��(1.75a��10-5)/b ��>
����������1�����������ᣬ���ڵ���ƽ�⣬����Ũ��Խ����̶�ԽС������0.1mol/L�Ĵ�����Һ�е�c(H+) ��0.01mol/L c(H+)�ı�ֵС��10��
��2���ٴ���ĵ��뷽��ʽΪCH3COOH CH3COO����H�������Ը��ݴ���ĵ���ƽ�ⳣ������ʽ
CH3COO����H�������Ը��ݴ���ĵ���ƽ�ⳣ������ʽ ��֪��������Ũ����c1(H+)=
��֪��������Ũ����c1(H+)=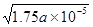 ��
��
�ڸ��� ��֪����ʱ������Ũ����
��֪����ʱ������Ũ����
c2(H+)=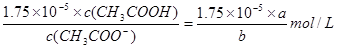 ��
��
�����ڴ���������ƴ���ĵ��룬����c1(H+)���� c2(H+)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺
��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺ ��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺
��ѧѧ���е�ƽ��������Ҫ��������ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ�����֣��Ҿ�������������ԭ������ش��������⣺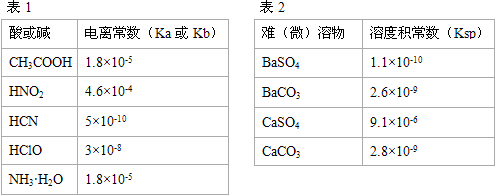
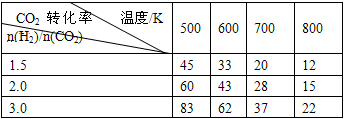
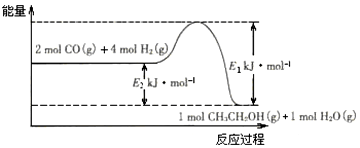
 CH3CH2OH(g)+3H2O(g) ��H��a kJ/mol ��һ��ѹǿ�£����������Ӧ��ʵ���������±���
CH3CH2OH(g)+3H2O(g) ��H��a kJ/mol ��һ��ѹǿ�£����������Ӧ��ʵ���������±���