��Ŀ����
A��B��C��D��E��Ԫ�����ڱ���ǰ36��Ԫ�أ��˵������������A��B���γ������Ļ����Dԭ���гɶԵ���������δ�ɶԵ�������3����E+�����е������ó���K��L��M�������Ӳ㡣
��1����̬Cԭ�Ӻ�����_____���˶�״̬��ͬ�ĵ��ӣ�Ԫ��C����̬�⻯��Ŀռ乹��Ϊ____��
��2��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________������Ԫ�ط��ű�ʾ��
��3��A��B�γɵĻ�����B2A2��Bԭ�ӵ��ӻ���ʽΪ____�������к��еĦҼ��ͦм������ֱ���______��_______��
��4��D����Ԫ���γɵ�ԭ����֮��Ϊl:1�������о��еĻ�ѧ������Ϊ______��
��5��E+��C�ļ������γɾ���ľ����ṹ��ͼ1��ʾ��ͼ�а����ʾ_______��
��6��E�ĵ��ʾ���ľ����ṹ��ͼ2��ʾ����ռ�������Ϊ_____��Բ�����æб�ʾ��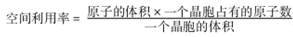 ��
��

���и���ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����� | ʵ������ | ���� |
A | Ũ�������Ҵ�170�湲�ȣ��Ƶõ�����ͨ������KMnO4��Һ | ��Һ��ɫ��ȥ | �Ƶõ�����Ϊ��ϩ |
B | �ⶨ��Ũ�ȵ�Na2CO3��Na2SO3����Һ��pH | ǰ��pH�Ⱥ��ߵĴ� | �ǽ����ԣ�S��C |
C | ��2.0mlŨ�Ⱦ�Ϊ0.1mol��L-1��KCl��KI�����Һ�еμ�1~2��0.01mol��L-1AgNO3��Һ���� | �����ʻ�ɫ | Ksp��AgCl����Ksp��AgI�� |
D | �����Һ���ȵμ�Ba(NO3)2��Һ�� �ٵμ�ϡ���� | ���ְ�ɫ���� | ԭ����Һ��һ������SO42- |
A. A B. B C. C D. D