��Ŀ����
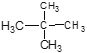
����Ŀ�����������(NH2COONH4)��һ���ֽ⡢��ˮ��İ�ɫ���壬������CCl4��ʵ���ҿɽ����������̼���ﰱ��ͨ��CCl4�н����Ʊ�����ѧ����ʽΪ��2NH3(g)+CO2(g)=NH2COONH4(s) ��H��0��
�ش��������⣺
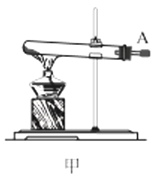
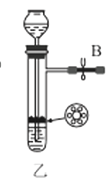

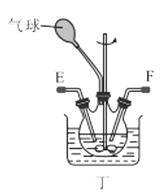
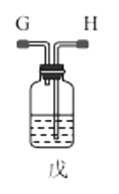
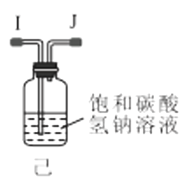
��1������װ�ü��Ʊ������Ļ�ѧ����ʽΪ__��
��2���������װ���������ԵIJ���__��
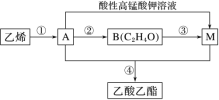
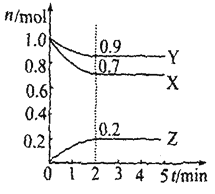
��3��ѡ��ͼ�е�װ���Ʊ���������泥������ӿڵ�����˳��Ϊ��B��__��__��EF��__��A��
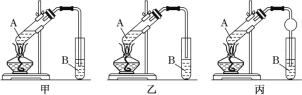
��4����ӦʱΪ�����Ӱ�������淋IJ���������ƿ�ļ��ȷ�ʽΪ__��������ˮԡ��������ˮԡ���������������������__��
��5����װ�ö��Ļ�����з������Ʒ�ķ�����__����д�������ƣ���
��6��ȡ����������Ϊ̼����淋İ����������Ʒ11.730g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ��������������Ϊ15.000g������Ʒ�а�������淋���������Ϊ__����֪��Mr(NH2COONH4)=78��Mr(NH4HCO3)=79��Mr(CaCO3)=100������������3λ��Ч���֣���
���𰸡�Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O �н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ�棬���ã���Һ���ֲ��䣬��������������� I J H G D(C)��C(D) ��ˮԡ ƽ����ѹ���ռ���������� ���� 79.8%
CaCl2+2NH3��+2H2O �н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ�棬���ã���Һ���ֲ��䣬��������������� I J H G D(C)��C(D) ��ˮԡ ƽ����ѹ���ռ���������� ���� 79.8%
��������
(1)ʵ�������Ȼ�狀��������ƾ��干����ȡ������
(2)�н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ��仯�����
(3)ѡ��ͼ�е�װ���Ʊ���������泥���ȡ�Ķ�����̼�к���HCl��ˮ��������ȡ����ʱ�����к���ˮ������Ҳ��Ҫ��ȥ�������壻
(4)���������(NH2COONH4)��һ���ֽ⡢��ˮ��İ�ɫ���壬������ʵ����ʷ������������������ƽ������ѹǿ��
(5)������֪��Ϣ�����������(NH2COONH4)������CCl4������
(6)ȡ����������Ϊ̼����淋İ����������Ʒ11.730g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ��������������Ϊ15.000g��ͨ��̼ԭ���غ㣬������Ʒ�а�������淋�����������
(1)ʵ�������Ȼ�狀��������ƾ��干����ȡ����������ʽΪ��Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
(2)���װ���������ԵIJ������н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ�棬���ã���Һ���ֲ��䣬��������������ã�
(3)ѡ��ͼ�е�װ���Ʊ���������泥���ȡ�Ķ�����̼�к���HCl��ˮ������Ӧ��ͨ��װ�����еı���̼��������Һ��ȥHCl��Ȼ��ͨ�����е�Ũ�����ȥˮ��������ȡ����ʱ�����к���ˮ������ͨ��װ�ñ��еļ�ʯ�ң������ӿڵ�����˳��Ϊ��B��I J��HG��EF������DC��CD��A��
(4)���������(NH2COONH4)��һ���ֽ⡢��ˮ��İ�ɫ���壬���ɰ�������淋ķ�Ӧ�Ƿ��ȵģ�������Ҫ�����¶ȣ�����ˮԡ���������������ƽ������ѹǿ���������ն���δ��Ӧ�����壻
(5)���������(NH2COONH4)����ˮ��İ�ɫ���壬������CCl4�������ʹ�ù��˵ķ������룻
(6)ȡ����������Ϊ̼����淋İ����������Ʒ11.730g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ��������������Ϊ15.000g��̼��Ƶ����ʵ���=![]() =0.1500mol������̼ԭ���غ㣬�����������Ʒ��̼�����ʵ���ҲΪ0.1500mol��̼����狀Ͱ�������淋����ʵ����ֱ�Ϊx��y����x+y=0.15��79x+78y=11.73����ã�x=0.03mol��y=0.12mol������Ʒ�а�������淋���������Ϊ
=0.1500mol������̼ԭ���غ㣬�����������Ʒ��̼�����ʵ���ҲΪ0.1500mol��̼����狀Ͱ�������淋����ʵ����ֱ�Ϊx��y����x+y=0.15��79x+78y=11.73����ã�x=0.03mol��y=0.12mol������Ʒ�а�������淋���������Ϊ![]() ��100%=79.8%��
��100%=79.8%��