��Ŀ����
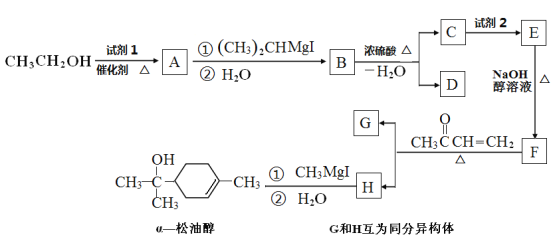
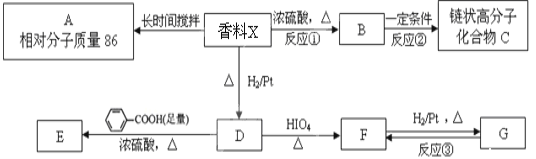
����Ŀ���ۺ���F�ĺϳ�·��ͼ���£�
��ݴ˻ش�
��1��A�к���������������_______��C��ϵͳ����Ϊ_________��
��2������B���������������õ��Լ���______��E��F�ķ�Ӧ������___________��
��3��C����D�ķ�Ӧ��ѧ����ʽΪ_____________________________�����C��NaOH�Ĵ���Һ��Ӧ�������ɵ��л���Ľṹ��ʽΪ________��ͬһ��̼ԭ����������̼̼˫���ij��⣩��
��4��G������![]() ��Ϊͬϵ���G���ʵ���Է���������
��Ϊͬϵ���G���ʵ���Է���������![]() ��14�ģ����������������G��ͬ���칹����____�֡�
��14�ģ����������������G��ͬ���칹����____�֡�
�� �����к��б������ұ�����������ȡ���� �� ���Ȼ�����Һ����ɫ
�� ������ˮ�����ӳɷ�Ӧ
��5�� ���������ϳ�·�ߣ����һ���ɼ�ȩ����ȩ�ͼ״�Ϊ��Ҫԭ���Ʊ� ![]() �ĺϳ�·��__________________��
�ĺϳ�·��__________________��
���𰸡�ȩ��1��2-�������NaHCO3��Һ(��Na2CO3)����ˮ����Br2��CCl4��Һ���Ӿ۷�ӦCH3CHBrCH2Br+2NaOH��CH3CH(OH)CH2OH+2NaBrCH3C��CH9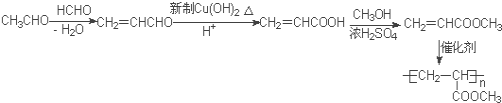
��������
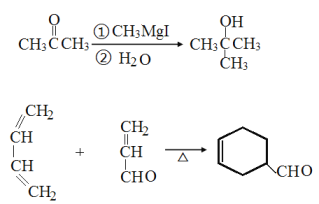
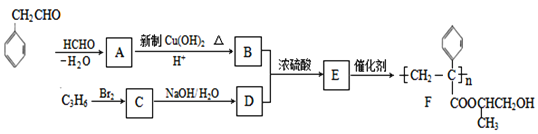
�������и�����ת����ϵ,���������Ϣ,����ȩ���ȩ������ȩ���ϲ�ʧˮ����A,A�Ľṹ��ʽΪ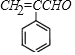 ��A������������ͭ����Һ��Ӧ,ȩ����Ϊ�Ȼ�,��B�Ľṹ��ʽΪ
��A������������ͭ����Һ��Ӧ,ȩ����Ϊ�Ȼ�,��B�Ľṹ��ʽΪ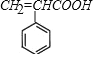 ����F�Ľṹ��ʽ����֪��,C3H6Ϊ��ϩ,CΪCH3CHBrCH2Br,��ˮ������D,DΪCH3CH(OH)CH2OH��B��D��Ũ���������·���������ӦӦ,���ɵ�EΪ
����F�Ľṹ��ʽ����֪��,C3H6Ϊ��ϩ,CΪCH3CHBrCH2Br,��ˮ������D,DΪCH3CH(OH)CH2OH��B��D��Ũ���������·���������ӦӦ,���ɵ�EΪ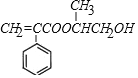 ��E�����Ӿ۷�Ӧ����F���ݴ˷��������
��E�����Ӿ۷�Ӧ����F���ݴ˷��������
�������и�����ת����ϵ,���������Ϣ,����ȩ���ȩ������ȩ���ϲ�ʧˮ����A,A�Ľṹ��ʽΪ ��A������������ͭ����Һ��Ӧ,ȩ����Ϊ�Ȼ�,��B�Ľṹ��ʽΪ
��A������������ͭ����Һ��Ӧ,ȩ����Ϊ�Ȼ�,��B�Ľṹ��ʽΪ ����F�Ľṹ��ʽ����֪��, C3H6Ϊ��ϩ,CΪCH3CHBrCH2Br,��ˮ������D,DΪCH3CH(OH)CH2OH��B��D��Ũ���������·���������ӦӦ,���ɵ�EΪ
����F�Ľṹ��ʽ����֪��, C3H6Ϊ��ϩ,CΪCH3CHBrCH2Br,��ˮ������D,DΪCH3CH(OH)CH2OH��B��D��Ũ���������·���������ӦӦ,���ɵ�EΪ ,E�����Ӿ۷�Ӧ����F��
,E�����Ӿ۷�Ӧ����F��
(1)AΪ ,��������������Ϊȩ������F�Ľṹ��ʽ����֪��, C3H6Ϊ��ϩ�������巢���ӳɷ�Ӧ���ɵ�CΪ1,2-�����������ˣ�������ȷ����:ȩ����1,2-���������
,��������������Ϊȩ������F�Ľṹ��ʽ����֪��, C3H6Ϊ��ϩ�������巢���ӳɷ�Ӧ���ɵ�CΪ1,2-�����������ˣ�������ȷ����:ȩ����1,2-���������
(2)BΪ ,B�к��еĹ�����Ϊ�Ȼ���̼̼˫��,����̼��������Һ�����Ȼ�������ˮ����̼̼˫����EΪ
,B�к��еĹ�����Ϊ�Ȼ���̼̼˫��,����̼��������Һ�����Ȼ�������ˮ����̼̼˫����EΪ ,Eͨ���Ӿ۷�Ӧ����F����ˣ�������ȷ����: NaHCO3��Һ(��Na2CO3)����ˮ����Br2��CCl4��Һ�����Ӿ۷�Ӧ��
,Eͨ���Ӿ۷�Ӧ����F����ˣ�������ȷ����: NaHCO3��Һ(��Na2CO3)����ˮ����Br2��CCl4��Һ�����Ӿ۷�Ӧ��
(3)CΪ1,2-�����������ˮ������D��DΪ1,2-���ǻ���������Ӧ�Ļ�ѧ����ʽΪ: CH3CHBrCH2Br+2NaOH��CH3CH(OH)CH2OH+2NaBr��C���������ƵĴ���Һ������ȥ��Ӧ����CH3C��CH����ȷ����CH3CHBrCH2Br+2NaOH��CH3CH(OH)CH2OH+2NaBr �� CH3C��CH��
(4)G������![]() ��Ϊͬϵ��,��G���ʵ���Է���������
��Ϊͬϵ��,��G���ʵ���Է���������![]() ��14,��G��
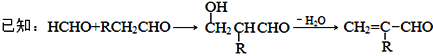
��14,��G��![]() ��1��CH2ԭ������������:�������к��б���,�ұ�����������ȡ�����������Ȼ�����Һ����ɫ,���л�������к��з��ǻ�����������ˮ�����ӳɷ�Ӧ,˵��������к���̼̼˫�������ݷ�������֪���������������л�������к��б���������������ȡ�����ֱ�Ϊ-OH ���CC3H5 �����CC3H5�ṹ����Ϊ��-CH=CHCH3��-CH2CH=CH2��-C(CH3)=CH2�����ǻ�����ȡ�����ڻ��ϵĽṹ��3�֣����ǻ�����ȡ�����ڻ��ϵĽṹ��3�֣����ǻ�����ȡ�����ڻ��ϵĽṹ��3�֣����������������л����ܹ�����3��3=9������ˣ�������ȷ����:9��
��1��CH2ԭ������������:�������к��б���,�ұ�����������ȡ�����������Ȼ�����Һ����ɫ,���л�������к��з��ǻ�����������ˮ�����ӳɷ�Ӧ,˵��������к���̼̼˫�������ݷ�������֪���������������л�������к��б���������������ȡ�����ֱ�Ϊ-OH ���CC3H5 �����CC3H5�ṹ����Ϊ��-CH=CHCH3��-CH2CH=CH2��-C(CH3)=CH2�����ǻ�����ȡ�����ڻ��ϵĽṹ��3�֣����ǻ�����ȡ�����ڻ��ϵĽṹ��3�֣����ǻ�����ȡ�����ڻ��ϵĽṹ��3�֣����������������л����ܹ�����3��3=9������ˣ�������ȷ����:9��
(5)����֪��Ӧ����֪������ȩ����ȩ������ȩ���Ϸ�Ӧ��ʧˮ����CH2=CHCHO��Ȼ����������������������ͭ��ȩ������Ϊ�Ȼ�������״���������Ӧ����CH2=CHCOOCH3���䷢���Ӿ۷�Ӧ�ɵ�![]() ����ϳ�·��Ϊ��
����ϳ�·��Ϊ�� 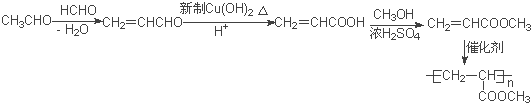 ����ȷ����
����ȷ����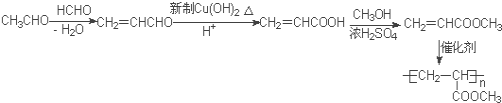 ��
��

����Ŀ����֪�������ݣ�
�� �� | �۵�/�� | �е�/�� | �ܶ�/g��cm��3 |
�� �� | ��114 | 78.4 | 0.79 |
�� �� | 16.6 | 117.9 | 1.05 |
�������� | ��83.6 | 77.5 | 0.900 |
ŨH2SO4 | 338 | 1.84 |



ʵ������ȡ������������Ҫװ������ͼI��ʾ����Ҫ����Ϊ������30mL�Ĵ��Թ��а������2��3��2�ı�������Ũ���ᡢ�Ҵ�������Ļ��Һ���ڰ���ͼI����װ�ã�ʹ����������������ͨ��ʢ��10mL����Na2CO3��Һ��(����2�η�̪��Һ)�Թ��У���С������Թ��еĻ��Һ���ܴ�С�Թ����ռ�Լ2mL����ʱֹͣ���ȣ�����С�Թܲ�������Ȼ���ã��ݷ����������������������ش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ�����_____________________________��
��2��д����ʵ����ȡ���������Ļ�ѧ����ʽ_________________________________��ŨH2SO4�������� _______________________��
��3��������У���С������Թ��еĻ��Һ����ԭ��_________________________��
��4����������۲쵽��������___________________________________________________
��5��������У���������������ķ�����_________________________________��
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ����������ͼ�ס��ҵ�װ��(��ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡԲ����ƿ�в���)������Ϊ����װ�ø�������Ϊʲô��_____��