��Ŀ����
����Ŀ��̼�����׳ƴ������;�ܹ㡣ʵ�����У���̼����狀ͱ���ʳ��ˮ���Ƶô��
(1)����ʳ��ˮ���õ�ԭ�ϴ�����Ҫ�����ں��Ӻ�ˮ�еõ���������ͨ���õķ�����_______��
(2)���õĴ���(��Ca2+��Mg2+��SO42-)��Ҫ�ᴿ����Ҫ��������NaOH��BaCl2��Na2CO3��Һ���������ӳ�ȥ�������˲����������pH��7����������BaCl2������Na2CO3��ԭ����______��
(3)����ʳ��ˮ�������ƴ��Ҳ���ȼҵ��ԭ�ϡ��ȼҵ�е�ⱥ��ʳ��ˮ�ķ���ʽΪ_______��
(4)���Դ����Ʒ��NaHCO3�����ķ����ǣ���_______(��������)��ȡ������Ʒ1.144g��������ˮ�ܽ⣬����0.500mol/L��HCl��Һ100mL���ټ�1-2�η�̪��������0.500mol/L������������Һ�ζ������ζ�����Һ��_____ɫ��_____ɫ������Ӳ���ɫΪ�ζ��յ㡣
(5)�����������й�������������58.00mL��������Ʒ��NaHCO3��������Ϊ_____��(����3λС��)
(6)���װ�������Ƶĵζ��ܵζ���������ʱ��©Һ������ᵼ�²ⶨ���______��ѡ����ƫ��������ƫ С����������Ӱ��������
���𰸡������� Na2CO3���Գ�ȥ������ BaCl2��������Na2CO3����ͨ�������ȥ 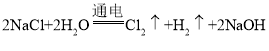 ������ƽ �� �� 0.073 ƫ��
������ƽ �� �� 0.073 ƫ��
��������
(1)����NaCl�ܽ�����¶�Ӱ��仯���������
(2)��ȥ���ʲ��������µ����ʣ��������������ʺ�Ҫ���׳�ȥ��
(3)��ⱥ��NaCl��Һ��Ӧ����NaOH��Cl2��H2��
(4)ȷ����һ�������Ĺ����õ�����ƽ���������ᣬHCl��Na2CO3��NaHCO3��Ӧ����NaCl������������÷�̪Ϊָʾ�����μ�NaOH��Һʱ������HCl��NaOH��Ӧ����NaCl��ˮ���ζ��ﵽ�յ�ʱ����Һ����ɫ��Ϊ��ɫ��
(5)�������ĵ�HCl��NaOH�������������NaHCO3�������������ɵô���Ĵ��ȣ�
(6)������Һ����������ʵ�����Ӱ������жϡ�
(1)����NaCl��ˮ�е��ܽ�����¶�Ӱ��仯����NaCl�Ӻ�ˮ�з���������ɲ��������ܼ��ķ�����
(2)��õ��ĺ�Ca2+��Mg2+��SO42-�Ĵ�����Ҫ�ᴿ���ȼ�����Ҫ��������BaCl2��Һ��ʹSO42-�γ�BaSO4�������ټ���Na2CO3��Һ���ȿ��Գ�ȥNaCl��Һ�е�Ca2+�γ�CaCO3������Ҳ���Խ�������BaCl2�γ�BaCO3�������ټ���NaOH��Һ��ʹMg2+�γ� Mg(OH)2������������Na2CO3����ͨ�������ȥ��
(3)�ڹ�ҵ��ͨ�����õ�ⱥ��NaCl��Һ�ķ�����ȡ����Ӧ����ʽΪ��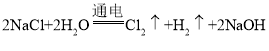 ��
��
(4)ȷ����һ�������Ĺ����õ�����ƽ���������ᣬHCl����Ʒ�е�Na2CO3��NaHCO3��Ӧ����NaCl�����������������÷�̪Ϊָʾ�����μ�NaOH��Һʱ������HCl��NaOH��Ӧ����NaCl��ˮ���ζ��ﵽ�յ�ʱ����Һ����ɫ��Ϊ��ɫ��
(5)�������⣬�����HCl��Na2CO3��NaHCO3��NaOH���ղ���NaCl��������Ʒ��Na2CO3��NaHCO3�����ʵ����ֱ���x��y������Ԫ���غ�ɵ�2x+y+n(NaOH)=n(HCl)����2x+y= n(HCl)-n(NaOH)=0.500mol/L��(0.1-0.058)L=0.021mol�����������غ�ɵ�106x+84y=1.144g����ʽ�������ɵ�x=0.01mol��y=0.001mol��������Ʒ��NaHCO3������Ϊm(NaHCO3)=0.001mol��84g/mol=0.084g��������Ʒ��NaHCO3��������Ϊ(0.084g��1.144g)��100%=0.073��
(6)���װ�������Ƶĵζ��ܵζ���������ʱ��©Һ�������Ӧ���ĵ�NaOH���ƫ��ʹ����Ʒ��Na2CO3������ƫС����NaHCO3������ƫ�����ջᵼ�²ⶨ���ƫ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�