��Ŀ����
����Ŀ��ij�Ż��������Ҫ�ɷ���FexS(SΪ-2��)���Ⱥ���Fe2+�ֺ���Fe3+����һ�����ĸôŻ�������100 mL������ǡ����ȫ��Ӧ(ע:��ʯ�������ɷֲ������ᷴӦ)������2.4 g���ʡ�0.425 mol FeCl2��һ����H2S���壬����Һ����Fe3+��������˵����ȷ���� (����)
A. 100 mL��������HCl�����ʵ���Ũ��Ϊ7.5 mol��L-1
B. ���ɵ�H2S�����ڱ�״���µ����Ϊ2.24 L
C. �ôŻ�����FexS�У�x=0.85
D. �ôŻ�����FexS�У�Fe2+��Fe3+�����ʵ���֮��Ϊ3��1
���𰸡�C
��������
n��S��=![]() =0.075mol������ת�Ƶ����غ��n��Fe3+��=
=0.075mol������ת�Ƶ����غ��n��Fe3+��=![]() =0.15mol����n��Fe2+��=0.425mol-0.15mol=0.275mol������Fe2+��Fe3+�����ʵ���֮��=0.275mol��0.15mol=11��6��
=0.15mol����n��Fe2+��=0.425mol-0.15mol=0.275mol������Fe2+��Fe3+�����ʵ���֮��=0.275mol��0.15mol=11��6��
A������ǡ�÷�Ӧ����FeCl20.425mol��������ԭ���غ��c��HCl��=![]() =8.5mol/L����A����
=8.5mol/L����A����
B��������ԭ�ӡ���ԭ���غ��n��H2S��=![]() n��HCl��=n��FeCl2��=0.425mol��V��H2S��=0.425mol��22.4L/mol=9.52L����B����
n��HCl��=n��FeCl2��=0.425mol��V��H2S��=0.425mol��22.4L/mol=9.52L����B����
C��FexS��n��S��=0.075mol+0.425mol=0.5mol��n��Fe��=0.425mol������n��Fe����n��S��=0.425mol��0.5mol=0.85������x=0.85����C��ȷ��
D������ת�Ƶ����غ��n��Fe3+��=![]() =0.15mol����n��Fe2+��=0.425mol-0.15mol=0.275mol������Fe2+��Fe3+�����ʵ���֮��=0.275mol��0.15mol=11��6����D����
=0.15mol����n��Fe2+��=0.425mol-0.15mol=0.275mol������Fe2+��Fe3+�����ʵ���֮��=0.275mol��0.15mol=11��6����D����
��ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����һ���ء��������Ԫ�صĻ�����������������̫���ܵ�ص���Ҫ���ϡ���֪��SeO2�ڳ������ǰ�ɫ�ľ��壬�۵�Ϊ340-350�棬��SeO2��������____���壬SeO2��Seԭ�Ӳ�ȡ���ӻ�����Ϊ___��
������A��X��Y��Z��W��M��G��ԭ������һ�������ǰ������Ԫ��
Ԫ�� | �����Ϣ |
A | ԭ�Ӻ����������������������ͬ |
X | ����̬ԭ�ӵ�L������3��δ�ɶԵ��� |
Y | ��̬ԭ�ӵ�2p�������һ�����ӵ�����������2p������������ӵ����������෴ |
Z | ԭ�Ӻ���p��������s��������l�� |
W | ԭ�ӵĵ�һ�����ĵ�����(kJ��mol-1)�ֱ��ǣ�I1=578��I2=1817 I3��2745 I4=11575 |
M | Ԫ�ص������������������4 |
G | ���̬ԭ������������Ϊ1����������Ӳ���������� |
��1������W��̬ԭ�ӵĺ�������Ų�ͼ______��
��2��A2Y��VSEPRģ������Ϊ____��
��3����֪ΪX2Y������Yԭ��ֻ��һ��Xԭ������������ݵȵ���ԭ����д��X2Y�ĵ���ʽ____��
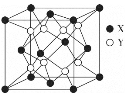
��4��X��G�γɵ�ij�ֻ�����ľ����ṹ��ͼ��ʾ�����仯ѧʽΪ______��
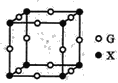
��5����֪Z��M�γɵĻ�����ľ����ܶ�Ϊpg��cm-3�������ӵ���ЧΪNA���þ������������������Z�������ļ����Ϊ_____cm��