��Ŀ����
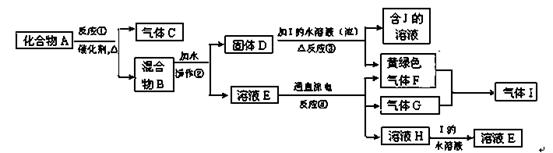
ij����С�����Mg��CO2�ķ�Ӧԭ����̽��Mg��NO2�ķ�Ӧ����������С��ͨ��ʵ��ȷ��Mg����NO2��ȼ�գ����Թ������������ּ��裺
I.����Ϊ���������ΪMgO II.����Ϊ��______________III.����Ϊ��______________
��ش��������⣺������Ϣ��2NO2+2NaOH=NaNO3+NaNO2+H2O

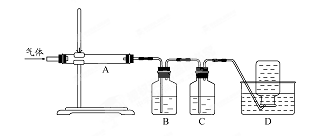
��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_____________________________
��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�___________________
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
��3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ����_________________________
��4��E���ռ��������������������ܶ���14,��������__________________
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������__________________������C�з����Ļ�ѧ��Ӧ����ʽ��_______________________________________________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��_______________________________________��
I.����Ϊ���������ΪMgO II.����Ϊ��______________III.����Ϊ��______________
��ش��������⣺������Ϣ��2NO2+2NaOH=NaNO3+NaNO2+H2O

��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_____________________________
��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�___________________
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
��3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ����_________________________
��4��E���ռ��������������������ܶ���14,��������__________________
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������__________________������C�з����Ļ�ѧ��Ӧ����ʽ��_______________________________________________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��_______________________________________��
��.�������ΪMg3N2 ��.�������ΪMgO��Mg3N2 ��1���رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á� ��2���ڢ� ��3���ž�װ���п�������ֹ��������ʵ�顣
��4��N2 ��5������� 4Mg+2NO2 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2
Mg3N2
��6������K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
��4��N2 ��5������� 4Mg+2NO2
 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2
Mg3N2��6������K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
�����������NO2�к���N��OԪ�أ�����Mg������ȼ�ղ��������MgO��Mg3N2�����ǵĻ��������.�������ΪMg3N2 ����.�������ΪMgO��Mg3N2��1����ͼ���Ӻ�������װҩƷǰװ�õ������Լ��鷽���ǣ��رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á���2��Cu��Ũ���ᷴӦ����������NO2���������壬����ʹ�ü��Ը����������ų� �ۼ�ʯ�ң�U�ι�װ���ǹ�����������Ũ������Һ�壬����ʹ�ã��ų���Ũ���ᡣ��װ��B��ʢװ�ĸ���������Ǣ���ˮCaCl2 �����������ס���3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ�����ž�װ���п�������ֹ��������ʵ�顣��4��E���ռ��������������������ܶ���14,��Է�������Ϊ14��2=28����������N2.��5������ȫת��ΪMgO����Ӧ��Ĺ��������Ƿ�Ӧǰ��(24+16)��24=1.67��.����ȫת��ΪMg3N2����Ӧ��Ĺ��������Ƿ�Ӧǰ��(24��3+14��2)��(24��3)=1.39����ʵ��õ�����������������ʵ��ǰMg��������1.5�������Եõ�����MgO��Mg3N2�Ļ����ʼ�����������C�з����Ļ�ѧ��Ӧ����ʽ��4Mg+2NO2
 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2����6����ʵ���д�������ȱ�ݣ�����û��β������װ�á��Ľ���ʩ�ǻ���K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�2�ķ�Ӧ���������ijɷ֡�
Mg3N2����6����ʵ���д�������ȱ�ݣ�����û��β������װ�á��Ľ���ʩ�ǻ���K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�2�ķ�Ӧ���������ijɷ֡�
��ϰ��ϵ�д�
�����Ŀ

 CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��
CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��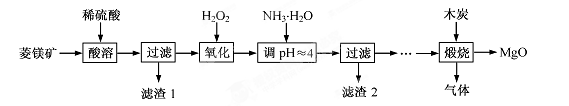
 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��