��Ŀ����
ijУ�о���ѧϰС�飬�������װ������֤Ũ������ľ̿�ڼ��������µIJ����к���SO2��CO2��ͬѧ�Dz������ϵ�֪���������ʹ���Ը��������Һ��ɫ����ѧ����ʽΪ��2KMnO4+5SO2+2H2O=K2SO4+2MnSO4+2H2SO4
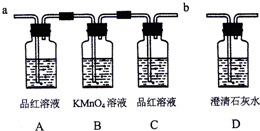
��1��ʵ��ʱ����Ӧ����������Ӧ��
��2��Aƿ��ʵ��������
��3��������ijͬѧ��Ϊ���γ���Ʒ����Һ����Ҫ������Ϊ
��4��Ҫ֤�������к���CO2��ʵ�������ǣ�C��Ʒ����Һ

��1��ʵ��ʱ����Ӧ����������Ӧ��
a
a
��ͨ��װ�ã����á�a����b����գ���2��Aƿ��ʵ��������
Ʒ����Һ��ɫ
Ʒ����Һ��ɫ
��Aƿ��Һ�������������������Ĵ���
�����������Ĵ���
��Bƿ��Һ�����������ն�����������
���ն�����������
����3��������ijͬѧ��Ϊ���γ���Ʒ����Һ����Ҫ������Ϊ
����
����
����ܡ����ܡ���ȥ��Cƿ��Cƿ��Һ������������������������Ƿ����
����������������Ƿ����
����4��Ҫ֤�������к���CO2��ʵ�������ǣ�C��Ʒ����Һ
����ɫ
����ɫ
��D�г���ʯ��ˮ�����
�����
����������1����Ӧ���ﺬ�ж�����̼�Ͷ����������壬��Ҫ����֤��������Ĵ��ڣ���ȥ������������֤������̼����Ĵ��ڣ�����ͨ����Һ������һ�������̳���
��2��A����֤������������Ĵ��ڣ�����������ʹƷ����Һ��ɫ��Bƿ�dz�ȥ����������������̼���岻�ܸ��ŵ�װ�ã�
��3������ȥ����C�Ǽ�����������Ƿ������װ�ã���Cװ�ò�����֤������̼�Ĵ��ڣ�
��4����Cװ��Ʒ�첻��ɫ��Dװ���� ����ʯ��ˮ�������ô���ж�����̼��
��2��A����֤������������Ĵ��ڣ�����������ʹƷ����Һ��ɫ��Bƿ�dz�ȥ����������������̼���岻�ܸ��ŵ�װ�ã�
��3������ȥ����C�Ǽ�����������Ƿ������װ�ã���Cװ�ò�����֤������̼�Ĵ��ڣ�
��4����Cװ��Ʒ�첻��ɫ��Dװ���� ����ʯ��ˮ�������ô���ж�����̼��
����⣺��1����Ӧ���ﺬ�ж�����̼�Ͷ����������壬����ʹ����ʯ��ˮ����ǣ���Ҫ����֤��������Ĵ��ڣ���ȥ������������֤������̼����Ĵ��ڣ�����ͨ����Һ������һ�������̳�������ʵ��ʱ����Ӧ����������Ӧ��a�˽���װ�ã�
�ʴ�Ϊ��a��
��2��A����֤������������Ĵ��ڣ�����������ʹƷ����Һ��ɫ��Bƿ�dz�ȥ���������ڼ��������̼����ʱ���ܸ��ŵ�װ�ã�
�ʴ�Ϊ��Ʒ����ɫ�������������Ĵ��ڣ����ն����������壻
��3��������̼�Ͷ����������壬����ʹ����ʯ��ˮ����ǣ�C�Ǽ�����������Ƿ������װ�ã�����ȥ������Cװ�ò�����֤������̼�Ĵ��ڣ�
�ʴ�Ϊ�����ܣ�����������������Ƿ������
��4����Cװ��Ʒ�첻��ɫ��֤�������������������Dװ���г���ʯ��ˮ�����֤�����ж�����̼��
�ʴ�Ϊ������ɫ������ǣ�
�ʴ�Ϊ��a��
��2��A����֤������������Ĵ��ڣ�����������ʹƷ����Һ��ɫ��Bƿ�dz�ȥ���������ڼ��������̼����ʱ���ܸ��ŵ�װ�ã�
�ʴ�Ϊ��Ʒ����ɫ�������������Ĵ��ڣ����ն����������壻
��3��������̼�Ͷ����������壬����ʹ����ʯ��ˮ����ǣ�C�Ǽ�����������Ƿ������װ�ã�����ȥ������Cװ�ò�����֤������̼�Ĵ��ڣ�
�ʴ�Ϊ�����ܣ�����������������Ƿ������
��4����Cװ��Ʒ�첻��ɫ��֤�������������������Dװ���г���ʯ��ˮ�����֤�����ж�����̼��
�ʴ�Ϊ������ɫ������ǣ�
���������⿼�����������ʵ�ʵ����ƣ���Ӧ�����װ��ѡ���������ڵķ����ж��ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ijУ�о���ѧϰС������ͬ��С��ͭƬ��пƬΪ�缫�о�ˮ����أ��õ���ʵ�����������ʾ��
ijУ�о���ѧϰС������ͬ��С��ͭƬ��пƬΪ�缫�о�ˮ����أ��õ���ʵ�����������ʾ��