��Ŀ����
����Ŀ����̼����(Na2CO4)��һ�ֺܺõĹ�����������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ2Na2CO4��4HCl=4NaCl��2CO2����O2����2H2O�����۹�̼����һ�㶼����̼���ƣ�Ϊ�ⶨij��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȣ�ij��ѧ��ȤС������������ַ���ʵʩ��
����һ��![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
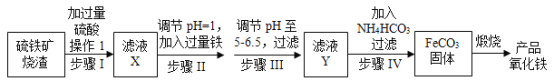
(1)�����ٺ͢۵����Ʒֱ�Ϊ______________________��
(2)���������У�ʹ�õ����������� _________________(��������)��
(3)����������۵IJ������̣�________________________________________________��
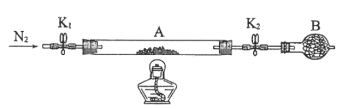
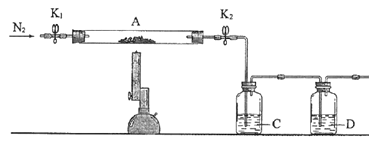
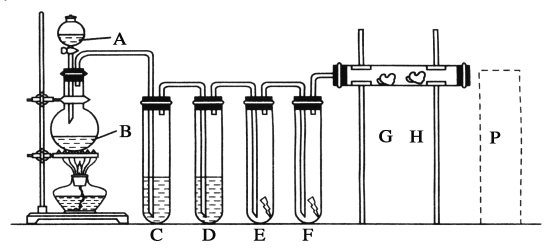
������������ͼ��ʾ��װ��ʵ��װ�ã�QΪһ��������������ȡ������Ʒ�����У���Һ©����������ϡ�����������������ַ�Ӧ��

(4)Ϊ�ⶨ��Ӧ������������������ϡ����ǰ����ر�______(����K1����K2������K3������ͬ)����________������A��������_____________________________________________��
(5)��������Ӧֹͣ��ʹK1��K3���ڹر�״̬��K2���ڴ�״̬���ٻ�����Kl�� B��װ�Ĺ����Լ���________________________��
(6)ʵ�����ʱ����Ͳ������x mLˮ����Ͳ�����ռ�����y mL���壬����Ʒ�й�̼���Ƶ�����������___________________��
���𰸡������������ᾧ �ڢ� �����������������г��ִ�������ʱ��ֹͣ���ȣ��������������������е�ʣ��ˮ�� K1��K2 K3 ƽ���Һ©���ںͷ�Ӧ��ϵ��ѹǿ��ʹϡ����˳�����£�ͬʱ��������ϡ�������������������Ӱ�� ��ʯ�� 122y/(53x-37y)
��������
�ⶨij��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȣ���Ʒ���������õ���Ʒ����Ϊm1g������ϡ�����ܽ����˵õ���ҺΪ�Ȼ�����Һ������Ũ���ᾧ�����Ȼ��ƣ������õ������Ȼ��Ƶ�����Ϊm2g�����ݷ���һ���̿��жϳ�������Ϊ������������Ϊ�ܽ⣬������Ϊ�����ᾧ������������Ͳ����ˮ�����Ϊ������CO2��O2���������Ͳ�����ռ���������ΪO2�����ݷ��������ݲⶨ����������������ʵ�������ϻ�ѧ����ʽ�����̼�������ʵ�������õ������������Դ˷������
���ݷ���һ���̿��жϳ�������Ϊ������������Ϊ�ܽ⣬������Ϊ�����ᾧ��
��1��������������֪������Ϊ������������Ϊ�����ᾧ��
�ʴ�Ϊ�������������ᾧ��
��2�������������õ������������ܽ�������ᾧ��
�ʴ�Ϊ���ڢۣ�
��3��������Ϊ�����ᾧ����������Ϊ�������������������г��ִ�������ʱ��ֹͣ���ȣ��������������������еĹ��壬
�ʴ�Ϊ�������������������г��ִ�������ʱ��ֹͣ���ȣ��������������������е�ʣ��ˮ�֣�
��4����Ӧ������CO2��O2ʹ����������ƿ�������ų���ˮ������Ͳ���У�������Ͳ����ˮ�������Ϊ������CO2��O2����������Ե�ϡ����ǰ����ر�K1��K2����K3������A��������ƽ���Һ©���ںͷ�Ӧ��ϵ��ѹǿ��ʹϡ����˳�����£�ͬʱ��������ϡ�������������������Ӱ�죬
�ʴ�Ϊ��K1��K2 ��K3 ��ƽ���Һ©���ںͷ�Ӧ��ϵ��ѹǿ��ʹϡ����˳�����£�ͬʱ��������ϡ�������������������Ӱ�죻
��5��Bװ��������ȥ���������е�CO2����B����װ�����Լ�Ϊ��ʯ�ҡ�������K1��Ϊ�������ɵ�CO2�ܹ���Bװ���м�ʯ�ҳ�����գ��Ӷ�ʹ��Ͳ���ռ����ϴ���O2��
�ʴ�Ϊ����ʯ�ң�
��6���������֪����Ͳ�����ռ���������ΪO2����Ͳ����ˮ��������ڷ�Ӧ������CO2��O2�����������ɽ������¼��㣺
n(Na2CO4)=2 n(O2)=![]() mmol��n(CO2)=
mmol��n(CO2)=![]() mmol��Na2CO4�����ᷴӦ����CO2�����ʵ���Ϊn(CO2)1=
mmol��Na2CO4�����ᷴӦ����CO2�����ʵ���Ϊn(CO2)1=![]() mmol����Na2CO3�����ᷴӦ����CO2�ļ�����ϵΪNa2CO3~CO2����n(Na2CO3)= n(CO2)2=
mmol����Na2CO3�����ᷴӦ����CO2�ļ�����ϵΪNa2CO3~CO2����n(Na2CO3)= n(CO2)2=![]() mmol��
mmol��
����Na2CO4����������w( Na2CO4)%=![]() 100%=122y/(53x-37y)��
100%=122y/(53x-37y)��
�ʴ�Ϊ��122y/(53x-37y)��

 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�