��Ŀ����
����Ŀ��ԭ������С��36��X��Y��Z��R��W����Ԫ�أ�����X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Y���γɻ�������������Ԫ�أ�Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�R����ռ���������![]() ��W��ԭ������Ϊ29���ش���������:
��W��ԭ������Ϊ29���ش���������:
��1��Y2X4������Yԭ�ӹ�����ӻ�����Ϊ________��1mol Z2X4���ЦҼ�����ĿΪ ________��
��2��������ZX3�뻯����X2R��VSEPR������ͬ�������幹�Ͳ�ͬ��ZX3�����幹��Ϊ ________�����ֻ���������л�ѧ���ļ��ǽ�С����________���÷���ʽ��ʾ����ͬ)��
��3����Rͬ��������ַǽ���Ԫ����X���γɽṹ���Ƶ��������ʣ����Ʋ����ߵ��ȶ����ɴ�С��˳��________�������� ________�����ߵķе��ɸߵ��͵�˳���� ________������ԭ��________��
��4��Ԫ��Y��һ����������Ԫ��Z�ĵ��ʻ�Ϊ�ȵ����壬Ԫ��Y������������ķ���ʽ��________��
��5��WԪ����________���˶�״̬��ͬ�ĵ��ӣ����̬ԭ�ӵļ۵����Ų�ʽΪ________��
���𰸡�sp2 5NA ������ H2O H2O >H2S> H2Se �뾶Se>S>O������H-Se> H-S> H-O������Խ�̣�����Խ����Խ�ȶ� H2O > H2Se>H2S H2O�γɷ��Ӽ������H2Se��Է�����������H2S�����Ӽ�������Խ���۷е�Խ�� CO 29 3d104s1
��������
ԭ������С��36��X��Y��Z��R��W����Ԫ�أ�����X�����ڱ��а뾶��С��Ԫ�أ���X��HԪ�أ�Y���γɻ�������������Ԫ�أ���Y��CԪ�أ�Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ���Z��NԪ�أ�R����ռ���������1/5��R��O��W��ԭ������Ϊ29����W��CuԪ�أ��ٽ�����ʽṹ�������
��1��C2H4����ƽ���νṹ������Cԭ�ӹ�����ӻ�����Ϊsp2�ӻ����������ǦҼ�����һ��N2H4�����к���5���Ҽ���
�ʴ�Ϊ��sp2 ��5NA��
��2��������NH3�뻯����H2O��VSEPR������ʣ����������壬�����幹�Ͳ�ͬ�����������к���1�Թ¶Ե��ӣ������幹��Ϊ�����Σ�ˮ�����к���2�Թ¶Ե��ӣ��������ֻ���������л�ѧ���ļ��ǽ�С����H2O��
�ʴ�Ϊ�������Σ�H2O��
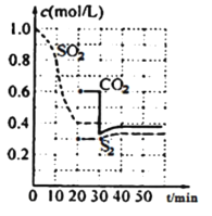
��3������ͬ������ϵ���ԭ�Ӱ뾶����������Խ�̣�����Խ������Խ�ȶ��������ȶ�����H2O��H2S��H2Se������H2O�γɷ��Ӽ������H2Se��Է�����������H2S�����Ӽ�������Խ���۷е�Խ�ߣ���е��С˳����H2O > H2Se>H2S��
�ʴ�Ϊ��H2O >H2S> H2Se���뾶Se>S>O������H-Se> H-S> H-O������Խ�̣�����Խ����Խ�ȶ���H2O > H2Se>H2S��H2O�γɷ��Ӽ������H2Se��Է�����������H2S�����Ӽ�������Խ���۷е�Խ�ߣ�
��4��Ԫ��C��һ����������Ԫ��N�ĵ��ʻ�Ϊ�ȵ����壬CO��N2��Ϊ�ȵ����壬����Ԫ��Y������������ķ���ʽ��CO��
�ʴ�Ϊ��CO��
��5��ͭԪ��ԭ��������29��������29���˶�״̬��ͬ�ĵ��ӣ����̬ԭ�ӵļ۵����Ų�ʽΪ3d104s1��
�ʴ�Ϊ��29 ��3d104s1��

����Ŀ���ڱ�״���½��мס��ҡ�������ʵ�飺��ȡ200mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһ��þ���Ͻ��ĩ���������壬�й����ݼ�¼���£�
ʵ����� | �� | �� | �� |
�Ͻ�����/g | 2.55 | 3.85 | 4.59 |
�����������/L | 2.80 | 3.36 | 3.36 |
�Իش�
(1)��������ʵ���Ũ��Ϊ________����������λ��Ч���֣�
(2)�Ͻ���þ�������ʵ���֮��_______��
����Ŀ�������ҹ����гɹ����漰�����У���Ҫ�ɷ�Ϊͬ����Ԫ���γɵ����ǽ������ϵ���
|
|
|
|
A��4.03�״�ھ�̼���跴�侵 | B��2022�궬�»�۰����ٻ��� | C�������ε�Ų���̼������������ | D�������ö������ѺϽ�ɸ���� |
A. AB. BC. CD. D