��Ŀ����
����Ŀ����ҵ����ij���̿���Ҫ�ɷ�ΪMnO2��������SiO2��Al2O3�����ʣ�Ϊԭ�ϣ������̵����е�SO2�Ʊ�MnSO4��H2O���������£�
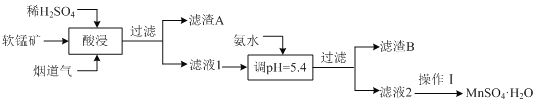
(1)����A����Ҫ�ɷ���_________���ѧʽ����
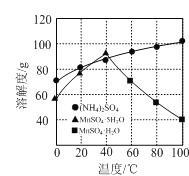
(2)������Ϊ���ȣ���У��ᾧ�����ȹ��ˡ�ϴ�ӡ����������ͼ�ܽ�����߷��������ȹ��˵�Ŀ�ij��˷�ֹMnSO4��H2O�к���(NH4)2SO4�⣬����____________________��
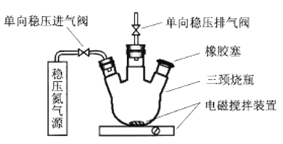
��3��MnSO4�����ڲ����ر�ˮ��DOֵ(ÿ��ˮ���ܽ���������������������)���ҹ����ر�ˮ�������������涨����������ˮԴ��DOֵ���õ���5 mg��L��1������ͬѧ���������ʵ�鲽��ⶨij��ˮ��DOֵ��
��һ����ʹ��ͼ��ʾװ���г���N2����ע������������ƿ�м���200 mLˮ����
�ڶ�������ע������������ƿ�����μ���һ����MnSO4��Һ��������������KI��Һ�����������������������������з�Ӧ��Mn2����O2��OH����MnO(OH)2����δ��ƽ��
�����������貢����ƿ�м���һ����H2SO4��Һ�������������£�����MnO(OH)2��I������ΪI2���䷴Ӧ���£� MnO(OH)2��I����H����Mn2+��I2��H2O��δ��ƽ��
���IJ�������ƿ��ȡ��40.00 mL��Һ����0.010 mol��L��1Na2S2O3��Һ������Ӧ��2S2O32����I2=S4O62����2I����ǡ����ȫ����ʱ������Na2S2O3��Һ4.40 mL��
�������Ƶڶ������������Լ�ʱ�������ܼ�ˮ������к�����ȴ����ʹ�ã����ܼ�ˮ��е�������_____��
��ͨ�������ж���Ϊ����ˮԴ���˺�ˮ��DOֵ�Ƿ���______ (д��������̣������ǵڶ������������Լ���ˮ������ı仯)��
���𰸡�SiO2 ����MnSO4��H2O����ʧ����ֹ����MnSO4��5H2O ��ȥˮ���ܽ������ ���
��������
������Ϣ��������֪�����̿�ͨ��ϡ������̵�������������������£������������������Ӧ���������̡���������ϡ���ᷴӦ���������������ˣ�����AΪ�������裬����Һ1�м��백ˮ������pH=5.4����Al3+ת��Ϊ�����������������ˣ�����BΪ������������Һ2Ϊ��������Һ���������ȣ���У��ᾧ�����ȹ��ˡ�ϴ�ӡ������MnSO4��H2O���ݴ�����
��1���ɷ�����֪����A����Ҫ�ɷ��Ƕ������裬��ѧʽΪ��SiO2���ʴ�Ϊ��SiO2��
��2�����ܽ�����߷�����֪�����ȹ��˵�Ŀ�ij��˷�ֹMnSO4��H2O�к���(NH4)2SO4�⣬���м���MnSO4��H2O����ʧ����ֹ����MnSO4��5H2O���ʴ�Ϊ������MnSO4��H2O����ʧ����ֹ����MnSO4��5H2O��
��3������Һ�������ܽ�Ȳ��������¶������ܽ�ȼ�С��������������������Һʱ��Ҫͨ������ܼ�����ȴ�����ܼ�ˮ���ܽ�������ϳ�������ʵ�����
�ʴ�Ϊ����ȥˮ���ܽ��������
��Mn2����O2��OH����MnO(OH)2�������ݹ۲취��ƽ��Ӧ����ʽΪ��2Mn2����O2��4OH-=2MnO(OH)2����MnO��OH��2+I-+H+��Mn2++I2+H2O����Ӧ�е�Ԫ�ػ��ϼ�-1�۱仯Ϊ0�ۣ���Ԫ�ػ��ϼ۽���+4�۱仯Ϊ+2�ۣ�����ת������2����ƽ�õ����ӷ���ʽΪ��MnO��OH��2+2I-+4H+=Mn2++I2+3H2O��
��Na2S2O3����Һ�ζ����ɵ�I2������Ӧ��2S2O32-+I2�TS4O62-+2I-���Ե�����ָʾ�����������������Һ���룬�ζ������е������һ����Һ��ɫ��Ϊ��ɫ�Ұ���Ӳ���˵����Ӧ�ﵽ�յ㡣
ǡ����ȫ����ʱ������Na2S2O3��Һ4.40mL��2Mn2++O2+4OH-�T2MnO��OH��2����MnO��OH��2+2I-+4H+=Mn2++I2+3H2O��2S2O32-+I2�TS4O62-+2I-���õ�������ϵΪ��
O2��2MnO��OH��2��2I2��4S2O32-��
1 4
n 0.0044L��0.010molL-1
n=1.1��10-5mol
200mLˮ���к�����1.1��10-5mol��200��40=5.5��10-5mol
����Ũ��=5.5��10-5mol��0.2L=2.75��10-4mol/L��
ˮ����������DO��=2.75��10-4mol/L��32g/mol=8.8��10-3g/L=8.8mg/L>5 mg/L����������ˮԴ��DO���ܵ���5mgL-1�����ˮ�������
�ʴ�Ϊ��ǡ����ȫ����ʱ������Na2S2O3��Һ4.40mL��2Mn2++O2+4OH-�T2MnO��OH��2����MnO��OH��2+2I-+4H+=Mn2++I2+3H2O��2S2O32-+I2�TS4O62-+2I-���õ�������ϵΪ��
O2��2MnO��OH��2��2I2��4S2O32-��
1 4
n 0.0044L��0.010molL-1
n=1.1��10-5mol
200mLˮ���к�����1.1��10-5mol��200��40=5.5��10-5mol
����Ũ��=5.5��10-5mol��0.2L=2.75��10-4mol/L��
ˮ����������DO��=2.75��10-4mol/L��32g/mol=8.8��10-3g/L=8.8mg/L>5 mg/L����������ˮԴ��DO���ܵ���5mgL-1�����ˮ����ꡣ



