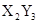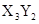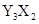��Ŀ����
��15�֣�X��Y��Z��W��R��TΪǰ������Ԫ����ԭ��������������Tԭ����������X��Y��Rԭ������֮�͡�ZΪ�ؿ��к�������Ԫ�ء�X��Zԭ�Ӻ������2��δ�ɶԵ��ӡ�Z��Rλ��ͬһ���塣X��Y��Z��W��R��T��ֻ�����ֽ���Ԫ�أ��Ҵ������з�Ӧ�� 2W+XZ2 X+2WZ
X+2WZ
�ش��������⣺
��1��X��Y��Z�ĵ�һ������������ ����Ԫ�ط��ű�ʾ����
��2����Ԫ��R��Ԫ��Z�γɵij����������У����ڷǼ��Է��ӵ��� ���ѧʽ�����÷���������ԭ���� �ӻ���
��3����X��TԪ����ɵĵ�����һ�������� ������ţ���
��4����̬Tԭ�ӵĺ�������Ų�ʽΪ ��
��5��T+����NH3ͨ����λ�����Ϊ[T(NH3)n]+����������T+��4s�����4p���ͨ��sp�ӻ�����NH3�ṩ�ŵ��Ӷԡ��� [T(NH3)n]+��n= ���� [T(NH3)n]+��T+��n����ԭ�ӹ��ɵĿռ�ṹ�� �͡�
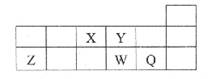
��6��������WZ��NaCl�ľ����ṹ����
���Ȼ��ƾ����ṹ����ͼ��ʾ����
����WZ�У������Ӻ������ӵ���λ����Ϊ ��
����֪WZ���ܶ�Ϊa g/cm3����WZ�о�������������Ӽ�ľ���Ϊ pm���ú�a����ʽ��ʾ������٤������ΪNA����
 X+2WZ
X+2WZ�ش��������⣺
��1��X��Y��Z�ĵ�һ������������ ����Ԫ�ط��ű�ʾ����
��2����Ԫ��R��Ԫ��Z�γɵij����������У����ڷǼ��Է��ӵ��� ���ѧʽ�����÷���������ԭ���� �ӻ���
��3����X��TԪ����ɵĵ�����һ�������� ������ţ���
| A�����Ӿ��� | B�����Ӿ��� | C��ԭ�Ӿ��� | D���������� |
��5��T+����NH3ͨ����λ�����Ϊ[T(NH3)n]+����������T+��4s�����4p���ͨ��sp�ӻ�����NH3�ṩ�ŵ��Ӷԡ��� [T(NH3)n]+��n= ���� [T(NH3)n]+��T+��n����ԭ�ӹ��ɵĿռ�ṹ�� �͡�
��6��������WZ��NaCl�ľ����ṹ����
���Ȼ��ƾ����ṹ����ͼ��ʾ����

����WZ�У������Ӻ������ӵ���λ����Ϊ ��
����֪WZ���ܶ�Ϊa g/cm3����WZ�о�������������Ӽ�ľ���Ϊ pm���ú�a����ʽ��ʾ������٤������ΪNA����
��1��N ��2��SO3��SP2 ��3��A ��4��1S22S22P63S23P63d104S1��[Ar] 3d104S1
��5��2��ֱ�� ��6��6��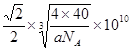
��5��2��ֱ�� ��6��6��
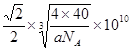
�����������1�����������֪�⼸��Ԫ�طֱ��ǣ�X��C��Y�� N��Z��O��W��Mg ;R��S��T��Cu����1��C��N��O��ͬһ���ڵ�Ԫ�أ�һ������£�Ԫ�صķǽ�����Խǿ��ԭ�Ӱ뾶ԽС�����һ������Խ��������Nԭ����������2p����ϵĵ��Ӵ��ڰ�������ȶ�״̬������N�ĵ�һ�����ܱ�O������˵�һ������������N����2��O��S����Ԫ�ؿ����γ�SO2��SO3���ֻ�������ڷǼ��Է��ӵ���SO3������Sԭ�ӵ��ӻ���ʽ��SP2����3��CԪ�صĵ����н��ʯ��ʯī��C60; ���ʯ��ʯī��ԭ�Ӿ��壬C60�Ƿ��Ӿ��壻Cu�γɵ��ǽ��������ڽ������塣��˲����ڵľ������������Ӿ��塣ѡ��ΪA����4����̬Cuԭ�ӵĺ�������Ų�ʽΪ1S22S22P63S23P63d104S1��[Ar] 3d104S1����5���� [CuNH3)n]+��n=2���� ��[Cu(NH3)n]+��Cu+��2����ԭ�ӹ��ɵĿռ�ṹ��ֱ���͡���6������MgO�У�ÿ��Mg2+������Χ��6��O2-��ÿ��O2-���ӵ���Χ��6��Mg2+���������ǵ���λ����Ϊ6������ÿ�������к��е�Mg2+�����ǣ�12��1/4+1=4�����е�O2-�����ǣ�8��1/8+6��1/2=4.���ÿ�������к���4��MgO�����辧���ĵı߳�ΪL�����ɾ����ṹʾ��ͼ��֪����������������Ӽ�ľ���Ϊ
 ��
��
��ϰ��ϵ�д�
�����Ŀ