��Ŀ����
9���л���A��̼���⡢������Ԫ����ɣ�Ϊ�о�A�������ṹ������������ʵ�飺��ȡA 10.4g������ʹ�������������ܶ�����ͬ������H2��52��������10.4gA��һ������������ȼ�գ����ᆳ��������Ũ�����Ũ�������������7.2g����ͨ������CuO��ַ�Ӧ������������ 3.2 g�����������ͨ����ʯ�ұ���ȫ���գ���ʯ����������17.6 g���л���A����������������Ӧ������Ԫ������B��B�ĺ˴Ź���������ֻ��һ�ַ���ͨ��������գ�
��1���л���A����Է�������Ϊ104
��2���л���A�ķ���ʽC4H8O3
��3��A�Ľṹ��ʽ
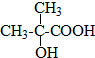 B�Ľṹ��ʽ
B�Ľṹ��ʽ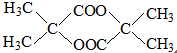 ��
��
���� ��1����ͬ�����£�������ܶ���Ħ�����������ȣ��ݴ�ȷ��A��Ħ����������Է�������
��2��Ũ�������ص�Ϊˮ����������ʯ�����ص�����Ϊ������̼���������ٸ���n=$\frac{m}{M}$����10.4gA��7.2gˮ��17.6g������̼�����ʵ��������������л�����Cԭ�ӡ�Hԭ�ӵ����ʵ������������֮�ͣ��ټ���Oԭ�ӵ����������ʵ����������Է�����������ȷ������ʽ��
��3���л���A�ɷ���������Ӧ������Ԫ������B��A�к���1���Ȼ���1���ǻ���B�ĺ˴Ź���������ֻ��һ�ַ壬B��ֻ��1�ֵ�ЧH��˵��B���жԳƽṹ����A�����в���H����ȫ��ͬ���ݴ�ȷ��A�Ľṹ��ʽ��b�Ľṹ��ʽ��
��� �⣺��1������ʹA���������ܶ�����ͬ������H2��52������A��Ħ������Ϊ��2g/mol��52=104g/mol����A����Է�������Ϊ104��
�ʴ�Ϊ��104��
��2��10.4g�л���A�����ʵ���Ϊ��$\frac{10.4g}{104g/mol}$=0.1mol��
Ũ�������ص�Ϊˮ����������0.1molA��ȫȼ������ˮ�����ʵ���Ϊ��$\frac{7.2g}{18g/mol}$=0.4mol��A�����к���H����ĿΪ��$\frac{0.4mol��2}{0.1mol}$=8��
���ռ�ʯ�����ص�����Ϊ������̼���������������̼�����ʵ���Ϊ��$\frac{17.6g}{44g/mol}$=0.4mol��A�����к���Cԭ�ӵ���ĿΪ��$\frac{0.4mol}{0.1mol}$=4��
A�����к���Oԭ����Ϊ��$\frac{104-12��4-8}{16}$=3��
�����л���A�ķ���ʽΪ��C4H8O3��
�ʴ�Ϊ��C4H8O3��
��3���л���A�ɷ���������Ӧ������Ԫ������B����A�����к���1��-OH��1��-COOH��B�ĺ˴Ź���������ֻ��һ�ַ壬��B��ֻ��1�ֵ�ЧHԭ�ӣ�˵��B���жԳƽṹ����A�����в���H�Ķ���ȫ��Ч�����A�ķ���ʽC4H8O3��֪��A��Ӧ�ú���2��������������������ͬһ��C�ϣ���A�Ľṹ��ʽΪ��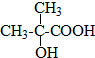 ��BΪ������Aͨ��������Ӧ���ɵģ���B�Ľṹ��ʽΪ��
��BΪ������Aͨ��������Ӧ���ɵģ���B�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ��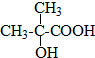 ��
�� ��
��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ�ע�����������غ㶨����ȷ���л������ʽ�е�Ӧ�ã���3��Ϊ�ѵ㣬��Ҫ��ȷ�����л���ṹ�����ʣ�

| A�� | ԭ�Ӱ뾶�Ƚϣ�X��Y��Z��W | |
| B�� | X���⻯��е�һ������Y���⻯�� | |
| C�� | Y���⻯���ȶ���һ������W���⻯�� | |
| D�� | ����������Ԫ���У�Z������������ˮ���������ǿ |
| A�� | ������Ԫ���γ����Ӻ��������Ӵﵽ���ﵽ8�����ȶ��ṹ | |
| B�� | ��������Ԫ�ص������ϼ����������������� | |
| C�� | ��3��4��5��6����Ԫ�ص���Ŀ�ֱ���8��18��32��32 | |
| D�� | ��4�����������ҵ�8��9��10������û�зǽ���Ԫ�� |
| A�� | �Ȼ�������ˮʱ���Ӽ�δ���ƻ� | B�� | ԭ�Ӿ����й��ۼ�Խǿ���۵�Խ�� | ||
| C�� | ���ڻ�ʱ�����й��ۼ��������� | D�� | ���Ӽ�������Խ����Խ�ȶ� |
 ת��Ϊ
ת��Ϊ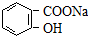 �ķ���Ϊ��������
�ķ���Ϊ��������| A�� | ��ϡH2SO4���Ⱥ���������NaOH | |
| B�� | ����������ϡ���Ṳ�� | |
| C�� | ��ϡH2SO4���Ⱥ���������NaHCO3 | |
| D�� | ��������NaOH��Һ���Ⱥ��ٵμӹ��������� |
| A�� | ��ʪ���pH��ֽ����Һ��pH | |
| B�� | ʵ�����Ʊ����﴿��������ʱ��������ͨ������ʳ��ˮ��ͨ��Ũ���� | |
| C�� | �ò������ڹ������Ͻ����Լ���AgCl������ϴ�� | |
| D�� | �к͵ζ�ʵ���У���ƿ������ˮϴ����δ����ⶨ���ƫ�� |
| A�� | c��H+�����٣��� | |
| B�� | �ֱ����1L 0.1mol•L-1HCl��Һ������Һ��pH���٣��� | |
| C�� | �ֱ�����Ũ�ȵ���������Һ�����ԣ����������������٣��� | |
| D�� | n��HF��+n��F-��=n��HClO��+n��ClO-�� |
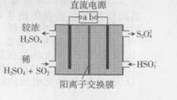 ������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ����NO2����������������ǣ�������
������ͼ��ʾװ�ã��缫��Ϊ���Ե缫��������SO2�����������ų�����Һ����NO2����������������ǣ�������| A�� | aΪֱ����Դ������ | |
| B�� | �����ĵ缫��ӦʽΪ��2HSO3-+e-=S2O42-+H2O | |
| C�� | �����ĵ缫��ӦʽΪ��SO2+2H2O-2e-=SO42-+4H+ | |
| D�� | ���ʱ��H+��������ͨ�������ӽ���Ĥ���������� |
| A�� | ��KSCN��Һ����Fe2+ | B�� | ��ʪ��ĺ�ɫʯ����ֽ���鰱�� | ||
| C�� | ��ʪ��ĵ��۵⻯����ֽ�������� | D�� | �������ữ��AgNO3��Һ����Cl- |