ƒøƒ⁄»ð
°æƒø°ø—«œıÀ·(HNO2) «“ª‘™»ıÀ·£¨≤ªŒ»∂®£¨÷ªƒÐ¥Ê‘⁄”⁄ΩœµÕŒ¬∂»µƒœ°»Ð“∫÷–°£ “Œ¬ ±£¨—«œıÀ·(HNO2)µƒµÁ¿Î∆Ω∫‚≥£ ˝Ka=5.1°¡10-4£¨H2CO3µƒµÁ¿Î∆Ω∫‚≥£ ˝Ka1=4.2°¡10-7°¢Ka2=5.61°¡10-11°£—«œıÀ·º∞∆‰—Œ‘⁄ µ—È∫Õ𧓵…˙≤˙÷–”–÷ÿ“™”¶”√°£«Îªÿ¥£∫
(1)‘⁄À·–‘Ãıº˛œ¬£¨NaNO2”ÎKI∞¥ŒÔ÷ µƒ¡ø1©U1«°∫√ÕÍ»´∑¥”¶£¨I£≠±ª—ıªØŒ™I2£¨–¥≥ˆ∏√∑¥”¶µƒ¿Î◊”∑Ω≥à Ω________________°£
(2)NaNO2»Ð“∫≥ _____–‘(ÃÓ°∞À·°±°∞ºÓ°±ªÚ°∞÷–°±)£¨‘≠“Ú «________(”√¿Î◊”∑Ω≥Ã Ω±Ì æ)°£“™µ√µΩŒ»∂®HNO2»Ð“∫£¨ø…“‘Õ˘¿‰∂≥µƒ≈®NaNO2»Ð“∫÷–º”»ÎªÚÕ®»Îƒ≥÷÷ŒÔ÷ £¨œ¬¡–ŒÔ÷ ≤ª ∫œ π”√______(ÃÓ–Ú∫≈)°£
a.ϡH2SO4 b.CO2 c.SO2
(3)»Ù”√µÁΩ‚∑®Ω´∑œÀÆ÷–NO2£≠◊™ªªŒ™N2≥˝»•£¨N2Ω´‘⁄__________(ÃÓµÁº´√˚≥∆)…˙≥…°£
(4)œÚ∫¨1 mol Na2CO3µƒ»Ð“∫÷–º”»Î1 mol HNO2∫Û£¨c(CO32-)°¢c(HCO3-)°¢c(NO2£≠)”…¥ÛµΩ–°µƒÀ≥–ÚŒ™________________°£
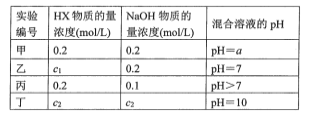
(5)25°Ê ±£¨”√0.100molL-1NaOH»Ð“∫µŒ∂®20.0mLƒ≥≈®∂»µƒHNO2»Ð“∫£¨»Ð“∫µƒpH”ÎNaOH»Ð“∫ê˝(V)µƒπÿœµ»ÁÕºÀ˘ 棨(‘⁄∏√Ãıº˛œ¬HNO2≤ª∑÷Ω‚)
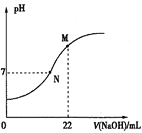
“—÷™£∫Mµ„∂‘”¶»Ð“∫÷–£¨c(OH£≠)=c(H+)+c(HNO2)°£‘Ú£∫
¢Ÿ‘≠»Ð“∫÷–c(HNO2)Œ™_________°£
¢⁄œ¬¡–πÿ”⁄Nµ„∂‘”¶»Ð“∫µƒÀµ∑®’˝»∑µƒ «______(ÃÓ—°œÓ◊÷ƒ∏)°£
A.»Ð÷ ÷ª”–NaNO2
B.ÀƵÁ¿Î≥ˆ¿¥µƒc(H+)=1°¡10-7 molL-1
C.»Ð“∫÷–£∫c(Na+)£ºc(OH-)
D.»Ð“∫÷–¿Î◊”≈®∂»£∫c(Na+)= c(NO2-)
°æ¥∞∏°ø4H+ + 2NO2£≠+ 2I£≠=I2 + 2NO°¸+ 2H2O ºÓ NO2£≠+H2OOH£≠+ HNO2 bc “ıº´ c(NO2£≠)> c(HCO3-) > c(CO32-) 0.110mol/L BD
°æΩ‚Œˆ°ø
(1)∏˘æð—ıªØªπ‘≠∑¥”¶÷–µ√ ßµÁ◊” ÿ∫„≈–∂œ∫¨N≤˙ŒÔ£¨Ω¯∂¯ È–¥¿Î◊”∑Ω≥Ã Ω£ª
(2)∏˘æð∏¥∑÷Ω‚∑¥”¶÷–«øÀ·÷∆»ıÀ·µƒ‘≠¿Ì“‘º∞—ıªØªπ‘≠∑¥”¶µƒ‘≠¿Ì≈–∂œ£ª
(3)µÁΩ‚≥ÿ÷–£¨“ıº´∑¢…˙µ√µÁ◊”µƒªπ‘≠∑¥”¶£¨—Ùº´∑¢…˙ ßµÁ◊”µƒ—ıªØ∑¥”¶£ª
(4) ◊œ»≈–∂œ∂˛’þ∑¥”¶µƒ≤˙ŒÔ£¨‘ŸΩ·∫œ‘Ω»ı‘ΩÀÆΩ‚µƒ‘≠¿Ì∑÷Œˆ£ª
(5)¢Ÿ∏˘æðMµ„∂‘”¶»Ð“∫÷–£¨c(OH£≠)=c(H+)+c(HNO2)£¨≈–∂œMµ„«°∫√…˙≥…NaNO2£¨æð¥Àº∆À„c(HNO2)£ª
¢⁄Nµ„»Ð“∫œ‘÷––‘£¨NaOH≤ª◊„£¨HNO2”– £”ý£¨»Ð÷ Œ™NaNO2°¢HNO2£¨æð¥À∑÷ŒˆΩ‚¥°£
(1)I£≠±ª—ıªØŒ™I2 ±£¨1mol I£≠ ßµÁ◊”1mol£¨NaNO2÷–N‘™ÀÿªØ∫œº€ «+3º€£¨”…”⁄‘⁄À·–‘Ãıº˛œ¬£¨NaNO2”ÎKI∞¥ŒÔ÷ µƒ¡ø1©U1«°∫√ÕÍ»´∑¥”¶£¨”…µ√ ßµÁ◊” ÿ∫„ø…÷™1molNaNO2µ√µÁ◊”1mol£¨‘ÚN‘™ÀÿªØ∫œº€”¶ΩµµÕŒ™+2º€£¨‘Ú≤˙ŒÔ÷–∫¨µ™µƒŒÔ÷ Œ™NO£¨≈‰∆Ω∏√¿Î◊”∑Ω≥Ã ΩŒ™4H+ + 2NO2£≠+ 2I£≠=I2 + 2NO°¸+ 2H2O£ªπ ¥∞∏Œ™£∫4H+ + 2NO2£≠+ 2I£≠=I2 +2NO°¸+2H2O£ª
(2)NaNO2Œ™«øºÓ»ıÀ·—Œ£¨NO2£≠∑¢…˙ÀÆΩ‚NO2£≠+H2OOH£≠+ HNO2£¨ π»Ð“∫≥ ºÓ–‘£ª“—÷™ “Œ¬ ±£¨—«œıÀ·(HNO2)µƒµÁ¿Î∆Ω∫‚≥£ ˝Ka=5.1°¡10-4£¨H2CO3µƒµÁ¿Î∆Ω∫‚≥£ ˝Ka1=4.2°¡10-7£¨‘ÚÀ·–‘£∫HNO2£æH2CO3£¨∏˘æð«øÀ·÷∆»ıÀ·µƒ‘≠¿Ìø…÷™£¨NaNO2°¢∂˛—ıªØú°¢ÀÆ≤ªƒÐ∑¥”¶…˙≥…HNO2£ª”…(1)÷™‘⁄À·–‘Ãıº˛œ¬£¨NaNO2ƒÐΩ´I£≠—ıªØŒ™I2£¨‘Ú≈®NaNO2»Ð“∫÷–Õ®»Î∂˛—ıªØ¡Ú ±£¨NaNO2ƒÐΩ´∂˛—ıªØ¡Ú—ıªØ£¨‘ÚŒÞ∑®µ√µΩHNO2£ªº”»Îœ°H2SO4£¨∑¢…˙∏¥∑÷Ω‚∑¥”¶…˙≥…HNO2£ªπ ¥∞∏Œ™£∫ºÓ£ªNO2£≠+H2OOH£≠+ HNO2£ªbc£ª
(3)”√µÁΩ‚∑®Ω´∑œÀÆ÷–NO2£≠◊™ªªŒ™N2≥˝»•£¨N‘™ÀÿªØ∫œº€ΩµµÕ£¨µ√µÁ◊”£¨∏˘æðµÁΩ‚≥ÿµƒ‘≠¿Ì£¨“ıº´∑¢…˙µ√µÁ◊”µƒªπ‘≠∑¥”¶£¨‘ÚN2Ω´‘⁄“ıº´…˙≥…£ªπ ¥∞∏Œ™£∫“ıº´£ª
(4)À·–‘£∫HNO2£æH2CO3£¨‘ÚœÚ∫¨1 mol Na2CO3µƒ»Ð“∫÷–º”»Î1 mol HNO2£¨∑¢…˙∑¥”¶£∫Na2CO3+ HNO2=NaHCO3+ NaNO2£¨‘Ú»Ð÷ ±‰Œ™1mol NaHCO3∫Õ1molNaNO2£¨”…”⁄À·–‘£∫HNO2£æH2CO3£¨∏˘æð‘Ω»ı‘ΩÀÆΩ‚‘≠¿Ì÷™£¨NO2£≠µƒÀÆΩ‚≥Ã∂»±»HCO3£≠µƒÀÆΩ‚≥Ã∂»–°£¨«“»ı¿Î◊”µƒÀÆΩ‚ «Œ¢»ıµƒ£¨HCO3£≠µƒµÁ¿Î «Œ¢»ıµƒ£¨‘Úc(CO32-)°¢c(HCO3-)°¢c(NO2£≠)”…¥ÛµΩ–°µƒÀ≥–ÚŒ™c(NO2£≠)>c(HCO3£≠) > c(CO32£≠)£ªπ ¥∞∏Œ™£∫c(NO2£≠)>c(HCO3£≠) > c(CO32£≠)£ª
(5)¢Ÿ“—÷™£∫Mµ„∂‘”¶»Ð“∫÷–£¨c(OH£≠)=c(H+)+c(HNO2)£¨‘ÚMµ„«°∫√…˙≥…NaNO2£¨”…¥Àø…÷™£¨22mL 0.100molL-1NaOH”Î20.0mL HNO2»Ð“∫«°∫√∑¥”¶£¨‘Úø…µ√c(NaOH)V(NaOH)=c(HNO2)V(HNO2)£¨0.022L°¡0.100molL-1=0.020Lc(HNO2)£¨Ω‚µ√c(HNO2)=0.110mol/L£ªπ ¥∞∏Œ™£∫0.110mol/L£ª
¢⁄Nµ„»Ð“∫œ‘÷––‘£¨NaOH≤ª◊„£¨HNO2”– £”ý£¨»Ð÷ Œ™NaNO2°¢HNO2£¨
A. »Ð÷ Œ™NaNO2°¢HNO2£¨AœÓ¥ÌŒÛ£ª
B. Nµ„»Ð“∫œ‘÷––‘£¨ÀƵÁ¿Î≥ˆ¿¥µƒc(H+)=1°¡10-7 molL-1£¨BœÓ’˝»∑£ª
C. Nµ„»Ð“∫œ‘÷––‘£¨»Ð÷ Œ™NaNO2°¢HNO2£¨c(Na+)‘∂¥Û”⁄c(OH-)£¨CœÓ¥ÌŒÛ£ª
D. Nµ„»Ð“∫œ‘÷––‘£¨”…µÁ∫… ÿ∫„£∫

 “ª≈µ È“µ ÓºŸ◊˜“µøÏ¿÷ºŸ∆⁄‘∆ƒœ√¿ ı≥ˆ∞Ê…Áœµ¡–¥∞∏
“ª≈µ È“µ ÓºŸ◊˜“µøÏ¿÷ºŸ∆⁄‘∆ƒœ√¿ ı≥ˆ∞Ê…Áœµ¡–¥∞∏°æƒø°ø«‚µ˛µ™À·(HN3)∫Õƒ™∂˚—Œ(NH4)2SO4°§FeSO4°§6H2O «¡Ω÷÷≥£”√‘≠¡œ°£
£®1£©∞±µ˛µ™À·“◊»Ð”⁄ÀÆ£¨25°Ê ±£¨∏√À·µƒµÁ¿Î≥£ ˝Œ™Ka=10°¡10-5
¢Ÿ«‚µ˛µ™À·‘⁄ÀƻГ∫÷–µƒµÁ¿Î∑Ω≥Ã ΩŒ™________________________________
¢⁄0.2mol/LµƒHN3»Ð“∫”Î0.1mol/LµƒNaOH»Ð“∫µ»Ãª˝ªÏ∫œ∫Û£¨ª÷∏¥µΩ25°Ê,ªÏ∫œ»Ð“∫÷–∏˜¿Î◊”∫ÕHN3∑÷◊”≈®∂»”…¥ÛµΩ–°µƒÀ≥–ÚŒ™__________________________°£
¢€“—÷™T°Ê ±£¨Ksp(CuN3)=5.0°¡10-9,Ksp(Cu2S)=2.5°¡10-48,‘ÚœýÕ¨Œ¬∂»œ¬£¨2CuN3(s)+S2-(aq)![]() Cu2S(s)+2N3-(aq)∏√∑¥”¶’˝∑¥”¶∑ΩœÚ_________(°∞ƒÐ°±ªÚ°∞≤ªƒÐ°±)Ω¯––ª˘±æ≥πµ◊£¨«ÎÕ®π˝º∆À„Àµ√˜_________________________°£
Cu2S(s)+2N3-(aq)∏√∑¥”¶’˝∑¥”¶∑ΩœÚ_________(°∞ƒÐ°±ªÚ°∞≤ªƒÐ°±)Ω¯––ª˘±æ≥πµ◊£¨«ÎÕ®π˝º∆À„Àµ√˜_________________________°£
£®2£©‘⁄FeSO4»Ð“∫÷–£¨º”»Î(NH4)2SO4πÃÃÂø…÷∆±∏ƒ™∂˚—Œæß㨌™¡À≤‚∂®≤˙∆∑¥ø∂»£¨≥∆»°ag≤˙∆∑»Ð”⁄ÀÆ£¨≈‰÷∆≥…500mL»Ð“∫£¨”√≈®∂»Œ™cmol/LµƒÀ·–‘∏þ√ÃÀ·ºÿ»Ð“∫µŒ∂®£¨√ø¥ŒÀ˘»°¥˝≤‚“∫ê˝æ˘Œ™25.00mL£¨ µ—ÈΩ·π˚º«¬º»Áœ¬£∫(“—÷™ƒ™∂˚—Œµƒ∑÷◊”¡øŒ™392)
µ—È¥Œ ˝ | µ⁄“ª¥Œ | µ⁄∂˛¥Œ | µ⁄»˝¥Œ |
œ˚∫ƒKMnO4»Ð“∫ê˝/mL | 25.52 | 25.02 | 24.98 |
µŒ∂®÷’µ„µƒœ÷œÛ «________________£¨Õ®π˝ µ—È ˝æ𣨺∆À„∏√≤˙∆∑µƒ¥ø∂»Œ™_________(”√∫¨◊÷ƒ∏a°¢cµƒ Ω◊”±Ì æ)°£…œ±Ìµ⁄“ª¥Œ µ—È÷–º«¬º ˝æð√˜œ‘¥Û”⁄∫Û¡Ω¥Œ£¨∆‰‘≠“Úø…ƒÐ «_________°£
A£Æµ⁄“ª¥ŒµŒ∂® ±£¨◊∂–Œ∆ø”√¥˝◊∞“∫»Ûœ¥
B£Æ∏√À·–‘∏þ√ÃÀ·ºÿ±Í◊º“∫±£¥Ê ±º‰π˝≥§£¨≤ø∑÷±‰÷
C£ÆµŒ∂®«∞À· ΩµŒ∂®πÐ÷–º‚◊Ï¥¶”–∆¯≈𣨵Œ∂®Ω· ¯∫Û∆¯≈ðœ˚ ß