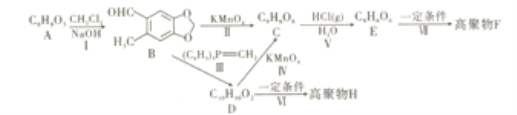
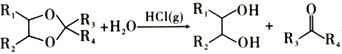��Ŀ����
����Ŀ����ͭ��(��Ҫ�ɷ�ΪCuFeS2)������ͭ�����������ԭ�ϡ��ش���������:
��1����̬Cuԭ�ӵļ۵����Ų�ʽΪ________
��2����ԭ�ӽṹ�Ƕȷ���,��һ������I1(Fe)��I1(Cu)�Ĺ�ϵ��:I1(Fe)____I1(Cu)(�>��<"��=��)
��3��Ѫ����������(C4H5N)����Ҫ�����Ѫ����(��Fe2+)����������ȱ����ƶѪ�����Ժ�Ѫ���صĽṹ����ͼ:
����֪�����еĸ���ԭ�Ӿ���ͬһƽ���ڣ�������������Nԭ�ӵ��ӻ�����Ϊ_______
��1mol���������������ĦҼ�����Ϊ____���������еĴ�м�����![]() ��ʾ������m���������γɴ�м���ԭ����,n���������γɴ�м��ĵ�����,�����Ի��еĴ�м�Ӧ��ʾΪ_____��
��ʾ������m���������γɴ�м���ԭ����,n���������γɴ�м��ĵ�����,�����Ի��еĴ�м�Ӧ��ʾΪ_____��
��C��N��O����Ԫ�صļ��⻯���У��е��ɵ͵��ߵ�˳��Ϊ________(�ѧʽ)��
��ѪҺ�е�O2����Ѫ�������������γɵ�Ѫ�쵰�������͵�,��Ѫ�쵰���е�Fe2+��O2��ͨ��_____�����ϡ�
��4����ͭ��ұ��ͭʱ������SO2�ɾ���SO2![]() SO3
SO3![]() H2SO4;���γ����ꡣSO2�Ŀռ乹 ��Ϊ________��H2SO4������ǿ��H2SO3��ԭ����____________
H2SO4;���γ����ꡣSO2�Ŀռ乹 ��Ϊ________��H2SO4������ǿ��H2SO3��ԭ����____________
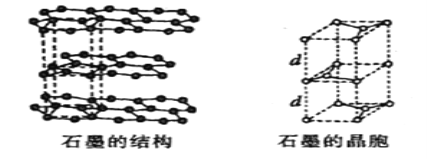
��5����ʯī���缫������ͭ����Ƶ�����ͭ��Һ�͵�����ʯī�ľ���ṹ����ͼ��ʾ,���߹��ճ������侧������ʯī�����к�̼ԭ����Ϊ____������֪ʯī���ܶ�Ϊ��g/cm3,C-C���ļ���Ϊrcm,�谢���ӵ�������ֵΪNA,��ʯī����IJ���d=______cm��
���𰸡� 3d104s1 �� sp2 10NA ![]() CH4��NH3��H2O ��λ�� V�� SO2(OH)2(��H2SO4)��S�Ļ��ϼ�Ϊ+6��S��������ǿ��SO(OH)2����H2SO3���е�S��ʹ�ǻ���O��H��Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ����������H+��������H2SO4ǿ��H2SO3 4
CH4��NH3��H2O ��λ�� V�� SO2(OH)2(��H2SO4)��S�Ļ��ϼ�Ϊ+6��S��������ǿ��SO(OH)2����H2SO3���е�S��ʹ�ǻ���O��H��Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ����������H+��������H2SO4ǿ��H2SO3 4 ![]()
����������1��Cuԭ�Ӻ�������Ų�Ϊ1s22s22p63s23p63d104s1����̬Cuԭ�ӵļ۵����Ų�ʽΪ.3d104s1 ����ȷ����.3d104s1��
2��Cuԭ�ӵļ۵���3d104s1��ʧȥ1�����Ӻ�3d10��Ϊȫ����״̬���ṹ�ȶ�������ͭԭ����ʧȥ1�����ӣ���һ�����ܽ�С������ԭ�Ӽ۵���Ϊ3d64s2��ʧȥ1�����Ӻ����ȶ��ṹ�����ԣ���ԭ�Ӳ���ʧȥ1�����ӣ���һ�����ܽϴ�����I1(Fe)>I1(Cu)����ȷ����>��
��3������֪�����еĵ�ԭ������������ԭ�Ӿ���ͬһƽ������Ϊƽ�������Σ�������������Nԭ�ӵ��ӻ�����Ϊsp2 ����ȷ����sp2��
�ڸ��ݷ��ӽṹ��֪1mol���������е�����Ϊ��������1��N-H������4��C-H������2��C-N������3��C-C������������������������Ϊ10 NA �����Ի��������γɴ�������ԭ����4��̼+1����=5����������Ϊ����ԭ����δ����ɼ��ĵ���Ϊ1�ԣ�̼̼ԭ�Ӽ�����γ������⣬����4��̼�ֱ��ṩ1�������γ����������е�����Ϊ6���������Ի��еĴ�����Ӧ��ʾΪ![]() ����ȷ�𰸣�10 NA ��
����ȷ�𰸣�10 NA �� ![]() ��
��
�����Ӽ���������е���ͣ���NH3��H2O�����о��������������ԭ�ӵĵ縺�Դ��뵪ԭ�����������H2O���Ӽ�ϴ�ˮ�ķе���ߣ�����������⻯��ķе��ɵ͵��ߵ�˳��ΪCH4��NH3��H2O����ȷ�𰸣�CH4��NH3��H2O��
��Fe2+�ṩ�չ����O2�ṩ�µ��Ӷ���ͨ����λ����������ȷ������λ����
��4��S��̬3s2 3p4��1��3s��2��3p����sp2�ӻ���SO2�Ŀռ乹��Ϊƽ����������SO2(OH)2(��H2SO4)��S�Ļ��ϼ�Ϊ+6��S��������ǿ��SO(OH)2����H2SO3���е�S��ʹ�ǻ���O��H��Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ����������H+��������H2SO4ǿ��H2SO3 ����ȷ����ƽ����������SO2(OH)2(��H2SO4)��S�Ļ��ϼ�Ϊ+6��S��������ǿ��SO(OH)2����H2SO3���е�S��ʹ�ǻ���O��H��Ĺ��õ��ӶԸ���ƫ��Oԭ�ӣ��ǻ����������H+��������H2SO4ǿ��H2SO3 ��
��5��ʯī�ľ����ṹ��ͼ��ʾ���辧���ĵױ߳�Ϊacm����Ϊhcm������dcm����h=2d����ͼ���Կ���1��ʯī�����к���4��̼ԭ��(4=8��![]() +4��
+4��![]() +2��
+2��![]() +1)��
+1)��
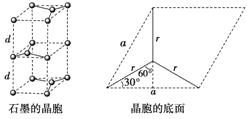
����ͼ��֪:a/2=r��sin 60�㣬��a=��3r��
��g��cm-3=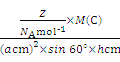 =
=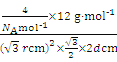 �����d=
�����d=![]() ����ȷ����4��
����ȷ����4��![]() ��
��

����Ŀ����ѧ�̲���ʾ��Ũ���������ˮ�ԡ���ˮ�ԡ�ǿ�����ԣ���ʹ���ۻ�����ijѧϰС��������и��ĸ����Ե�Ũ�����Ũ�ȷ�Χ������������ʵ��̽����
��1�����Ʋ�ͬŨ�ȵ�������18.4 mol/L��Ũ�������Ʋ�ͬŨ�ȵ����ᡣ���в�����ȷ����_____��
A. ��ȡŨ���� B.
��ȡŨ���� B.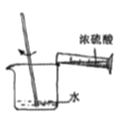 ϡ��Ũ���� C.
ϡ��Ũ���� C. ת��������ƿ D.
ת��������ƿ D. ����
����
��2��Ũ�������ˮ�ԡ���ˮ�ԡ�������Ũ�ȵĹ�ϵ
��Ũ�������ˮ��:��ȡ0.5g�����������Թ��У��ֱ����3mL��ͬŨ�ȵ����ᡣ
��Ũ�������ˮ��:��ȡһ��ľ�����Ա���,�ֱ����1mL��ͬŨ�ȵ����ᡣ
��Ũ����Ķۻ�:��ȡԼ1cm����ɰֽ��ĥ������˿,�����Թ��м���3mL��ͬŨ�ȵ����ᡣ
ʵ�������±�:
ʵ�� | c(H2SO4)/mol/L | 18.4 | 12 | 11 | 10 | 9 | 8 | 7 | 6 | 1-5 |
�� | ������ɫ�仯 | ��һ�� | ��һ�� | ��һ�� | �� | �� | �� | �� | �� | �� |
�� | ľ����ɫ�仯 | ��� | ��� | ��� | ��� | ��� | ��� | ��� | ��� | ���� |
�� | ��˿�������� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
��ϱ������ݻش���������:
�û�ѧ����ʽ��ʾ����������һ������ԭ��:_______;�������Ũ����______mol/Lʱ��������ˮ�ԡ�
��3��Ũ�����ǿ��������Ũ�ȵĹ�ϵ
���Թ��зֱ����1С��ͭƬ,�����Թ��зֱ����2mL ��ͬŨ�ȵ�����,����ͼ��ʾ��װ�ý���ʵ�顣(�г�������ȥ)
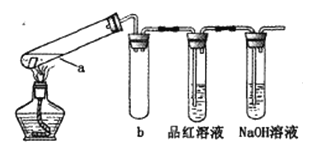
��bװ�õ�������________
�ڱ�ʵ����֤��Ũ�������ǿ�����Ե�������_________��________��
���Թ�a�м���ʱ������ɫ�Ĺ���,������ú�ɫ�����к���Cu2S��д�����ɸ����ʵĻ�ѧ����ʽ____.
�ܾ���ʵ�鷢��:c(H2SO4)�� 6mol/Lʱ,������ͭ�ڼ��ȷ�Ӧʱ���ɱ���ǿ�����ԡ���ͬѧԤ��,ͭƬ��5mol/L�������ڳ�ʱ���������ʱ��Ҳ�ᷢ����Ӧ����Ԥ���������_______.
��4���ۺϸ�С��ͬѧ��̽�����,��ѧ�̲���ͬʱ��������ˮ�ԡ���ˮ�ԡ�ʹ���ۻ���ǿ����������Ũ�����Ũ�ȷ�ΧΪ________mol/L��