��Ŀ����
����Ŀ��ʵ��������ૼ�ȩΪԭ���Ʊ�ૼ״���ૼ��ᡣ
���Ʊ�ԭ����
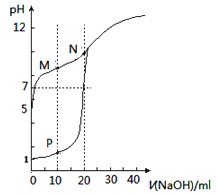
2![]() ��NaOH
��NaOH![]() ��
��![]()
![]() ��HCl
��HCl![]() ��NaCl
��NaCl
��ʵ�鲽��
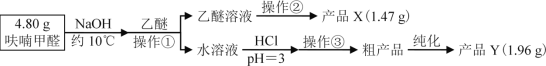
�������Ϣ
ૼ�ȩ | ૼ״� | ૼ��� | ���� | |
�۵�/�� | ��36.5 | ��29 | 133 | ��116.3 |
�е�/�� | 161.7 | 170 | 231 | 34.5 |
ˮ���� | �� | �� | ���� | ���� |
��Է������� | 96 | 98 | 112 | 74 |
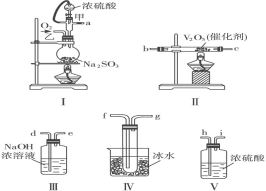
����ʵ��װ��
V��������˼�����ش��������⣺
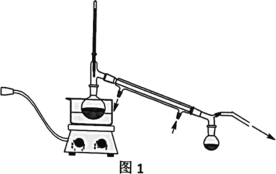
��1������������___
��2�������ڵ�װ����ͼ1��ʾ���ռ���ƷXʱ�¶ȼƵĶ���Ӧ������90�����ң���ԭ����___��
��3������������ˮ��Һ�������������pHΪ2��3��pH��3��������___��������ҺpHʱ��Ӧѡ���ָʾ����__��
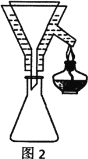
��4���ֲ�ƷY����������ͼ2װ�ý����ȹ��ˣ������������ͭ©���м���ˮ��___����װ�á��漰����˳���������ѡ�
A������©��֧��������̾�©����������ֽ���Ž�Һ�ձ��������ȵĴ���Һ
B������̾�©�����Ž�Һ�ձ�������©��֧����������ֽ�������ȵĴ���Һ
C������̾�©����������ֽ������©��֧�����Ž�Һ�ձ��������ȵĴ���Һ
D������̾�©����������ֽ���Ž�Һ�ձ��������ȵĴ���Һ������©��֧��
��5��������30mL��ȡ�����ѣ�����ȡЧ���Ƕ�˼��������4����ȡ��ʽ���������__��
A��30mL��0mL��0mL B��10mL��10mL��10mL
C��15mL��10mL��5 mL D��5mL��10mL��15mL
��6�������ƷY�IJ�����(Y)��___��
���𰸡���ȡ��Һ ૼ��� ��ѹ�����ૼ״��е㽵�ͣ�����170��(Լ90��)ʱ�����ռ�����ֹ�¶ȹ�������ૼ״��ṹ�ƻ� ����pH��3��ʹૼ�������ȫת��Ϊૼ�������� ����(��չ���) A B 70%
��������
ૼ�ȩ�ڼ��������·����绯��Ӧ����![]() ��
��![]() ��
��![]() ���������ѣ�����������ȡ��Һ���õ�
���������ѣ�����������ȡ��Һ���õ�![]() ��������Һ��
��������Һ��![]() ��ˮ��Һ��
��ˮ��Һ��![]() ������������õ�
������������õ�![]() ��
��![]() ��ˮ��Һ�������ᣬ����pH=3������
��ˮ��Һ�������ᣬ����pH=3������![]() �����������ؽᾧ���ᴿ
�����������ؽᾧ���ᴿ![]() ��
��
�������Ϸ�������1���������Ǽ������ѣ�����![]() ��������Һ��
��������Һ��![]() ��ˮ��Һ��������ȡ��Һ��
��ˮ��Һ��������ȡ��Һ��![]() ��ˮ��Һ�������ᣬ����pH=3������
��ˮ��Һ�������ᣬ����pH=3������![]() ���������Բ�ƷYΪૼ��ᡣ
���������Բ�ƷYΪૼ��ᡣ
��2��������Ϊ����װ�ã���ֹ�¶ȹ�������ૼ״��ṹ�ƻ���ͨ����ѹ�����ૼ״��е㽵�ͣ�����170��(Լ90��)ʱ�����ռ�ૼ״��������¶ȼƵĶ���Ӧ������90�����ҡ�
��3������������ˮ��Һ���������ᣬ����pH��3��ʹૼ�������ȫת��Ϊૼ��������������Ҫ����pH��3��������pH��3.1ʱ���ʺ�ɫ�����Կ�����ҺpHʱӦѡ�������ָʾ����
��4��Ϊ��ֹૼ���ᾧ�������ֲ�ƷY�����������ȹ��ˣ������������ͭ©���м���ˮ������©��֧��������̾�©����������ֽ���Ž�Һ�ձ��������ȵĴ���Һ����װ�á���ѡA��
��5������ȡЧ���Ƕ�˼������30mL��ȡ�����ѣ���÷ֶ�ε�����ȡ���ϲ���ȡҺ����ѡB��
��6��ૼ�ȩ�����ʵ�����![]() ������2
������2![]() ��NaOH
��NaOH![]() ��
��![]() ��
��![]() ��HCl
��HCl![]() ��NaCl������ૼ�������۲�����0.025mol��������0.025mol��112g/mol=2.8g��ʵ������ૼ����������1.96g����ƷY�IJ�����(Y)��
��NaCl������ૼ�������۲�����0.025mol��������0.025mol��112g/mol=2.8g��ʵ������ૼ����������1.96g����ƷY�IJ�����(Y)��![]() 70%��
70%��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�����Ŀ�����ڵؿ��еĺ����ϸߡ��輰�仯����Ŀ��������Ѿã����ִ��������й㷺Ӧ�á��ش��������⣺
��1��1810����仯ѧ�ұ�������˹�ڼ���ʯӢɰ��ľ̿����ʱ���õ�һ������������������������������_______��
��2���մɡ�ˮ��Ͳ����dz��õĹ����β��ϡ����У�������ͨ��������Ҫԭ����_______��
��3���ߴ������ִ���Ϣ���뵼��������Ȳ�ҵ����Ҫ�Ļ������ϡ���ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ����Ҫ��Ӧ���£�
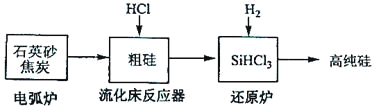
��������Ҫ��Ӧ | |
�绡¯ | SiO2+2C |
��������Ӧ�� | Si+3HCl |
��ԭ¯ | SiHCl3+H2 |
����ʯӢɰ�ͽ�̿�ڵ绡¯�и��¼���Ҳ��������̼���裬�÷�Ӧ�Ļ�ѧ����ʽΪ_______��̼�����ֳ�_______���侧��ṹ��_______���ơ�
������������Ӧ�IJ����У�SiHCl3��Լռ85%������SiCl4��SiH2Cl2��SiH3Cl�ȣ��й����ʵķе��������±����ᴿSiHCl3����Ҫ���ղ��������dz�����������_______��
���� | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | SiH4 |
�е�/�� | 2355 | 57.6 | 31.8 | 8.2 | -30.4 | -84.9 | -111.9 |
��SiHCl3����ˮ�⣬����ȫˮ��IJ���Ϊ_______��
��4���ȼҵ��Ϊ�������������ṩ����ԭ�ϣ���Щԭ����_______��
����Ŀ��Na��Al��Fe��Cu����ѧ��ѧ����Ҫ�Ľ���Ԫ�ء����ǵĵ��ʼ��仯����֮���кܶ�ת����ϵ���±��������ʲ��ܰ���ͼ(��������ʾһ�����)��ϵ�ת������
ѡ�� | A | B | C | D |
|
a | Na | Al | Fe | Cu | |
b | NaOH | Al2O3 | FeCl3 | CuO | |
c | NaCl | Al(OH)3 | FeCl2 | CuCl2 |
A.AB.BC.CD.D