��Ŀ����
ij����С��ͬѧ����ͼ��ʾװ�ý���ʵ�飨�г�װ����ʡ�ԣ���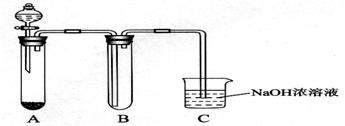
��1���ø�װ���Ʊ�NO2���о������ʡ�
���ռ�һ�Թ�NO2��ȡ���Թ�B������ˮ�У��۲쵽��������________________��
�ڸ÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
��C��NaOH��Һ����NO2�����������Σ��ұ��� ���뱻��ԭ��NO2�����ʵ���֮����1��1����д���÷�Ӧ�����ӷ���ʽ________________________________��
��2����װ��Ҳ�������Ʊ�Cl2���о������ʡ�
����A�й���ΪƯ�ۣ�����Ũ���ᣬ��A�з�����Ӧ�Ļ�ѧ����ʽ��___________��
����B�з�һС��ʪ��ĵ��۵⻯����ֽ���ɹ۲쵽��ֽ�������������ӷ���ʽ����ԭ��________________________________________��
����������ƣ�Na2S2O3�������NaOH��Һ������������֪25.0mL 0.1 mol��L-1��Na2S2O3��Һǡ�ðѱ�״����224 mL Cl2��ȫת��ΪCl��ʱ��S2O32��ת����_____����ѡ���
a. S2�� b. S c. SO32�� d. SO42��
��12�֣�ÿ��2�֣���1����Һ������������ɫ�����Ϊ��ɫ
��3NO2��H2O��2HNO3��NO ��2NO2��2OH����NO2����NO3����H2O
��2����Ca��ClO��2��4HCl��Ũ����CaCl2��2Cl2����2H2O
��Cl2��2I����2Cl����I2 I��������ΪI2�������۱��� ��d
���������������1����NO2����ˮ���������NO�������ռ�һ�Թ�NO2��ȡ���Թ�B������ˮ�У��۲쵽��������Һ������������ɫ�����Ϊ��ɫ��
�ڸ÷�Ӧ�Ļ�ѧ����ʽ��3NO2��H2O��2HNO3��NO��
�����ڱ������뱻��ԭ��NO2�����ʵ���֮����1��1���������ɵ������ƺ��������Ƶ����ʵ���֮����1:1�ģ���÷�Ӧ�����ӷ���ʽ��2NO2��2OH����NO2����NO3����H2O��
��2����Ư�۵���Ч�ɷ��Ǵ�����ƣ�������ƾ���ǿ�����ԣ��ܰ�Ũ��������������������Ӧ�Ļ�ѧ����ʽ��Ca��ClO��2��4HCl��Ũ����CaCl2��2Cl2����2H2O��
����������ǿ�����ԣ�I��������ΪI2�������۱�������Ӧ�����ӷ���ʽ��Cl2��2I����2Cl����I2��
��Na2S2O3��SԪ�صĻ��ϼ��ǣ�2�ۣ�������������SԪ�صĻ��ϼ���n������ݵ��ӵĵ�ʧ�غ��֪��0.025��0.1����n��2����2��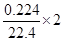 �����n��6�������������������ƣ���ѡd��
�����n��6�������������������ƣ���ѡd��
���㣺����NO2�����ʡ��������Ʊ���������ԭ��Ӧ���й��жϺͼ��㡢����ʽ����д
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij������͡�������۽̲ģ������߿��������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԡ�Ҳ����������ѧ���������������ͷ�ɢ˼ά����������ѧ����ѧ��������

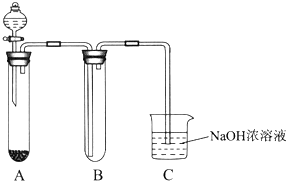 ij����С��ͬѧ����ͼ��ʾװ�ý���ʵ�飨�г�װ����ʡ�ԣ���
ij����С��ͬѧ����ͼ��ʾװ�ý���ʵ�飨�г�װ����ʡ�ԣ���



