��Ŀ����
����Ŀ��C��CuO�ڸ����·�Ӧ��������Cu��![]() ��
��![]() ��CO���ֽ�
��CO���ֽ�![]() ̼�۸�
̼�۸�![]() ��ϣ���Ӳ���Թ��и����������¼��ȣ������ɵ�����ȫ��ͨ������NaOH��Һ�У����ռ���������壬�����Һ���ӵ�����Ϊ
��ϣ���Ӳ���Թ��и����������¼��ȣ������ɵ�����ȫ��ͨ������NaOH��Һ�У����ռ���������壬�����Һ���ӵ�����Ϊ![]() �����������ڱ�״���µ����Ϊ
�����������ڱ�״���µ����Ϊ![]() ������˵������ȷ����
������˵������ȷ����
A.��Ӳ���Թ��и����������¼��ȹ�������ʱ����![]() ̼�μ��˷�Ӧ
̼�μ��˷�Ӧ
B.�Թ��з���������������ԭ��Ӧ��ת�Ƶ���![]()
C.��Ӧ���Թ���ʣ��Ĺ��������������Ϊ![]()
D.��Ӧ����ͭ��������ͭ�������ʵ���Ϊ![]()
���𰸡�D
��������
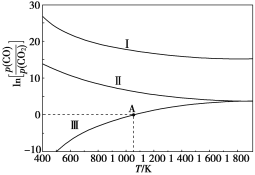
���ɵ�����ȫ��ͨ������NaOH��Һ���ռ��������塣�����Һ���ص�![]() Ϊ������̼��������
Ϊ������̼��������![]() �����ʵ���Ϊ��
�����ʵ���Ϊ��![]() �����������ڱ�״����560mL����ΪCO���������CO�����ʵ���Ϊ��
�����������ڱ�״����560mL����ΪCO���������CO�����ʵ���Ϊ��![]() ��
��
A.����̼Ԫ���غ��֪���μӷ�Ӧ��Cԭ�ӵ����ʵ�������![]() ��CO�����ʵ���֮�ͣ����Բμӷ�Ӧ��̼Ԫ������Ϊ��
��CO�����ʵ���֮�ͣ����Բμӷ�Ӧ��̼Ԫ������Ϊ��![]() ����A��ȷ��
����A��ȷ��
B.��Ӧ��CԪ�ػ��ϼ����ߣ�ͭԪ�ػ��ϼ۽��ͣ�����ת�Ƶ������ʵ���Ϊ��![]() ����B��ȷ��
����B��ȷ��
C.���ɵ�![]() ��CO��������Ϊ��
��CO��������Ϊ��![]() �����Է�Ӧ���Թ��й�������������Ϊ��
�����Է�Ӧ���Թ��й�������������Ϊ��![]() ����C��ȷ��
����C��ȷ��
D.����ͭ�����ʵ���Ϊ��![]() ��������̼��һ����̼���е���ԭ�����ʵ���Ϊ��
��������̼��һ����̼���е���ԭ�����ʵ���Ϊ��![]() ����Ӧ����ԭ�Ӵ�����������ͭ�У�����������ͭ�����ʵ���Ϊ��
����Ӧ����ԭ�Ӵ�����������ͭ�У�����������ͭ�����ʵ���Ϊ��![]() ��ͭ�����ʵ���Ϊ��
��ͭ�����ʵ���Ϊ��![]() ����Ӧ����ͭ��������ͭ�������ʵ���Ϊ
����Ӧ����ͭ��������ͭ�������ʵ���Ϊ![]() ����D����
����D����
�ʴ�ΪD��

 ��У����ϵ�д�
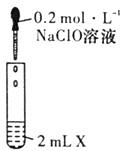
��У����ϵ�д�����Ŀ��NaClO��Ư��Һ����Ч�ɷ֣�ij�о�С��̽��NaClO��Һ�����ʣ����������ʵ�飺
װ��ͼ | �Լ�X | ʵ������ | |
|
|
| ��������ɫ���� |
|
| ��Һ���� | |
|
| ��Һ��� | |
|
| ������ɫ���� | |
�����жϲ���ȷ����![]()
![]()
A.ʵ��![]() �з�������Ҫ��Ӧ��
�з�������Ҫ��Ӧ��![]()
B.ʵ��![]() �з�������Ҫ��Ӧ��
�з�������Ҫ��Ӧ��![]()
C.ʵ��![]() �и�������������ǿ��
�и�������������ǿ��![]()
D.ʵ��![]() ��
��![]() ��
��![]() ��ٽ�ˮ��
��ٽ�ˮ��