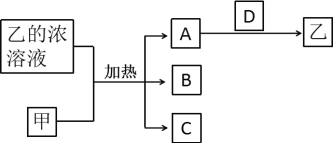
��Ŀ����
����Ŀ�������к������ᣬ�����ڳ��³�ѹ����һ���Ⱥܴ��Һ�塣ȡ9.0g������������Na��Ӧ���ڱ�״�����ռ���2.24L���壻��ȡ9.0g������������NaHCO3��Һ��Ӧ�����ɵ�CO2�����ڱ�״���µ����Ϊ2.24L����֪��������к���һ��������ش�
��1���������Է�������Ϊ___________________��
��2��������NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽΪ___________________��
��3����Ũ��������£��������������Ӧ���ɻ�״��������״���Ľṹ��ʽΪ_________
���𰸡�90 ![]()
![]()

��������
��OH����������Ʒ�Ӧ����COOHҲ�������Ʒ�Ӧ����OH������HCO3����Ӧ���ٽ�������Ϣ������2.24LCO2�������ʵ���Ϊ0.1mol����Ҫ0.1mol��COOH��0.1mol��COOH���Ʒ�Ӧֻ������11.2LH2�����ǹ�����22.4LH2��˵������0.1mol��OH����9.0g�����к���0.1mol�ǻ���0.1mol�Ȼ�����һ����������к���һ���ǻ���һ���Ȼ�����9.0g��������ʵ���Ϊ0.1mol�����������Է�������Ϊ90��������������к���һ��������ȷ������Ľṹ��ʽΪCH3CH(OH)COOH����һ����������к��������ǻ��������Ȼ�����9.0g��������ʵ���Ϊ0.05mol�����������Է�������Ϊ180��������������к���һ��������ʱ��д����������������Ľṹ��ʽ���������ƣ���ȷ���������Է���������Ϊ90����ṹ��ʽΪCH3CH(OH)COOH��
(1)���������ķ������������Է�������Ϊ90��
(2)��������еģ�COOH������HCO3����Ӧ�����ɣ�COONa��CO2��ˮ����ѧ����ʽΪCH3CH(OH)COOH��NaHCO3![]() CH3CH(OH)COONa��CO2����H2O��
CH3CH(OH)COONa��CO2����H2O��
(3)�ڷ���������Ӧ��ʱ����������е��Ȼ���ȥ�ǻ����ǻ���ȥ��ԭ�ӣ���һ���������ͬ�����γɻ�״��������ṹ��ʽΪ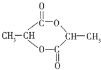 ��
��