��Ŀ����
����Ŀ����Ҫ����գ�
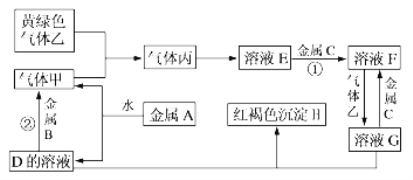
(1)�л���A��Ʒ3.0g������������²��������ȼ�յ�3.36 L CO2(��״��)��3.6 g H2O����������A����Է�������Ϊ60��A�ķ���ʽΪ____________��A�ڴ���Cu���������ܱ�����������C��C���ܷ���������Ӧ����A��������____________��C�й���������Ϊ____________��
(2)![]() ��ͬ���칹���У����ܷ���������Ӧ��������FeCl3��Һ������ɫ��Ӧ�Ĺ���________�֣����к˴Ź�������Ϊ5��壬�ҷ������Ϊ2��2��2��1��1��Ϊ_____________ (д�ṹ��ʽ)��
��ͬ���칹���У����ܷ���������Ӧ��������FeCl3��Һ������ɫ��Ӧ�Ĺ���________�֣����к˴Ź�������Ϊ5��壬�ҷ������Ϊ2��2��2��1��1��Ϊ_____________ (д�ṹ��ʽ)��
���𰸡�C3H8O 2-���� �ʻ� 13 ![]()
��������
(1) ��״���£�3.36 L CO2�����ʵ���=![]() =0.15mol��3.6 g H2O�����ʵ���=
=0.15mol��3.6 g H2O�����ʵ���=![]() =0.2mol����A�к�����Ԫ�ص�����m(O)=3.0g-(0.2mol��2��1g/mol) - (0.15mol��12 g/mol)=0.8g������n(O)=
=0.2mol����A�к�����Ԫ�ص�����m(O)=3.0g-(0.2mol��2��1g/mol) - (0.15mol��12 g/mol)=0.8g������n(O)=![]() =0.05mol��N(C)��N(H)��N(O)=0.15��0.4��0.05=3��8��1������A��ʵ��ʽΪC3H8O����������A����Է�������Ϊ60��A�ķ���ʽΪC3H8O��A�ڴ���Cu���������ܱ�����������C��C���ܷ���������Ӧ����AΪCH3CHOHCH3������Ϊ2-������A��������C��CΪCH3COCH3�����еĹ�����Ϊ�ʻ����ʴ�Ϊ��C3H8O��2-�������ʻ���
=0.05mol��N(C)��N(H)��N(O)=0.15��0.4��0.05=3��8��1������A��ʵ��ʽΪC3H8O����������A����Է�������Ϊ60��A�ķ���ʽΪC3H8O��A�ڴ���Cu���������ܱ�����������C��C���ܷ���������Ӧ����AΪCH3CHOHCH3������Ϊ2-������A��������C��CΪCH3COCH3�����еĹ�����Ϊ�ʻ����ʴ�Ϊ��C3H8O��2-�������ʻ���
(2)![]() ��ͬ���칹���У����ܷ���������Ӧ��������FeCl3��Һ������ɫ��Ӧ��˵���ṹ�к���ȩ���ͷ��ǻ�������Ϊ-CH2CHO��-OH�����ڡ��䡢��3�֣�����Ϊ-CH3��-CHO��-OH����-CH3��-CHO������λʱ��-OH��4��λ�ã���-CH3��-CHO���ڼ�λʱ��-OH��4��λ�ã���-CH3��-CHO���ڶ�λʱ��-OH��2��λ�ã��ʹ���13�֣����к˴Ź�������Ϊ5��壬�ҷ������Ϊ2��2��2��1��1�Ľṹ��ʽΪ
��ͬ���칹���У����ܷ���������Ӧ��������FeCl3��Һ������ɫ��Ӧ��˵���ṹ�к���ȩ���ͷ��ǻ�������Ϊ-CH2CHO��-OH�����ڡ��䡢��3�֣�����Ϊ-CH3��-CHO��-OH����-CH3��-CHO������λʱ��-OH��4��λ�ã���-CH3��-CHO���ڼ�λʱ��-OH��4��λ�ã���-CH3��-CHO���ڶ�λʱ��-OH��2��λ�ã��ʹ���13�֣����к˴Ź�������Ϊ5��壬�ҷ������Ϊ2��2��2��1��1�Ľṹ��ʽΪ![]() ���ʴ�Ϊ��13��
���ʴ�Ϊ��13��![]() ��
��