��Ŀ����
����Ŀ������ѧ����ѡ��3:���ʽṹ�����ʡ�
ͭ���Ͻ�����Ҫ������ң������������������Ʒ���ش��������⣺
��1��ͭԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_________��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ_________��
��2��������ͭ�백ˮ�����γ�[Cu(NH3)4](OH)2����ɫ��Һ��
��[Cu(NH3)4](OH)2�������ӵ����幹����_________��
����[Cu(NH3)4]2+��Cu2+��NH3֮���γɵĻ�ѧ����Ϊ_______________���ṩ�µ��ӶԵijɼ�ԭ����_______________��
�۰������γɵľ�������_______���壨������ͣ�����ṹ�еĵ�ԭ��ֻ���γ�_______�����������ԭ����______________________________________��
��3��Nԭ�ӵĵ�һ������IN=l402.3kJ/mol��0ԭ�ӵĵ�һ������I0=1313.9kJ/mol��IN>I0��ԭ����_____________________________��
��4������ͭ����������__________���γɵľ��塣
��5��ͭ���Ͻ�����������ṹ��ͼ��ʾ��

�پ�������ԭ����ͭԭ�ӵ�������Ϊ_______��
�����Ͻ���ܶ�Ϊdg/cm3������������D��Niԭ�ӵ����Cuԭ�ӵľ���Ϊ_______ cm����NAΪ�����ӵ�������ֵ����
���𰸡� 1s22s22p63s23p63d104s1��[Ar]3d104s1 0 ���ı��� ��λ�� N ���� 3 ��ԭ��ֻ��3��δ�ɶԵ��� Nԭ�ӵ�2p�Dz㣨2p3��Ϊ�����������ع����Dz�����ڰ�����ȫ����ȫ��ʱԭ�ӵ������ή�ͣ����ȶ���ʧ���ӽ��ѣ�����������Ҳ���֣� �������10������ 1��3 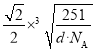 ��������������Ҳ���֣�
��������������Ҳ���֣�![]()
����������1��ͭԭ������Ϊ29����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1��3d�ܼ���û��δ�ɶԵ��ӡ�
��2����Cu2+���ӹ���Ϊ3d9��[Cu(NH3)4]2+Ҳ�ɱ�ʾΪCu(NH3)4(H2O)2]2+��Ϊsp3d2�ӻ���ͬʱ�������䣬�γ��Ķ�(Cu��NH3��λ��)����(H2O��Cu��λ��)��λ��������H2O��Cu��λ������������Ϊ�����ڣ����Ǿͳ���ƽ�����ı��νṹ����[Cu(NH3)4]2+�����У�Cu2+�����չ����NH3�ṩ�¶Ե��ӣ�Cu2+��NH3֮���γ���λ������NH3���ɷ��ӹ��ɵĹ��ۻ�����γɵľ������ڷ��Ӿ��壻Nԭ�ӵ����Ų�ʽΪ1s22s22p3��ֻ��3��δ�ɶԵ��ӣ�����ֻ���γ�3���������
��3��һ������£�Ԫ�ص�ԭ�Ӱ뾶ԽС��Ԫ�صķǽ�����Խǿ��������ܾ�Խ����Nԭ�ӵ�2p�Dz㣨2p3��Ϊ�����������ع����Dz�����ڰ�����ȫ����ȫ��ʱԭ�ӵ������ή�ͣ����ȶ���ʧ���ӽ��ѣ�����Nԭ�ӵĵ�һ�����ܴ���Oԭ�ӵĵ�һ��������
��4������ͭ�������ǽ�������������ͭ���������ɽ������γɵľ�����
��5���پ�����ͭԭ�������ģ���ԭ���ڶ��㣬���ݾ�̯�����㣬������ͭԭ�Ӹ���Ϊ6��![]() ��3����ԭ�ӵĸ���Ϊ8��
��3����ԭ�ӵĸ���Ϊ8��![]() ��1����ͭ����ԭ�Ӹ�����Ϊ3��1���ڸ������Ϸ������þ��������ΪCu3Ni�����Ͻ���ܶ�Ϊdg��cm-3��NAΪ�����ӵ�������ֵ�������ܶ���
��1����ͭ����ԭ�Ӹ�����Ϊ3��1���ڸ������Ϸ������þ��������ΪCu3Ni�����Ͻ���ܶ�Ϊdg��cm-3��NAΪ�����ӵ�������ֵ�������ܶ���![]() ����d=
����d=![]() ��������a��
��������a��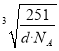 ��������ͼ��ʾ���辧���������D��Niԭ�ӵ����Cuԭ�ӵľ���Ϊx����2x2=a2������x=
��������ͼ��ʾ���辧���������D��Niԭ�ӵ����Cuԭ�ӵľ���Ϊx����2x2=a2������x=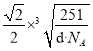 ��
��

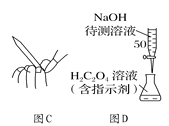
����Ŀ����֪ij���巴Ӧ��ƽ�ⳣ���ɱ�ʾΪK=c(CH3OCH3)c(H2O)/c2(CH3OH)���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ����400����K=32��500����K=44����ش��������⣺
��1��д��������Ӧ�Ļ�ѧ����ʽ��_________________________________ ��
��2���÷�Ӧ����H _________0��
��3����֪���ܱ������У����ijʱ�̸���ֵ�Ũ�����£�
���� | CH3OH(g�� | CH3OCH3(g�� | H2O(g�� |
Ũ��/��molL-1�� | 0.54 | 0.68 | 0.68 |
�ٴ�ʱ�¶�400�棬��ijʱ������_______���������������ͬ����
�������¶�Ϊ�����꣬�Ը��¶���ƽ��̬�״����ʵ���nΪ�����꣬��ʱ��Ӧ����ͼ���λ����ͼ��____�㣬�Ƚ�ͼ��B��D��������Ӧ������Ӧ������B_______��D��������____��

��4��һ��������Ҫ��߷�Ӧ���ת���ʣ����Բ��õĴ�ʩ��___________��
a�������¶� b��������� c��ѹ�����������
d������ˮ������Ũ�� e����ʱ���������