��Ŀ����
����ˮ�����ǹ�ϵ���ཡ������Ҫ���⡣(1)������������������ˮ���������ʣ�������������Ҫ����������ˮ�������˴����ᣬ�÷�Ӧ�����ӷ���ʽΪ__________________����������������̷����գ���Ӧ�����ӷ���ʽΪ____________________________________________________________________��
(2)������������ˮ����������ζ�⣬���������ܲ���500�������°����»����õ��л����������CHCl3��CCl2Br2�ȡ�д��CH4��Cl2��Ӧ����CHCl3�Ļ�ѧ����ʽ��
__________________��__________________��__________________��
���Ϸ�Ӧ������_________��Ӧ(���л���Ӧ����)���ɴ˿�֪��ÿ����1 mol CHCl3������Cl2�����ʵ���_________ (����ڡ������ڡ���С�ڡ�)3 mol��
(3)�ø�������(Na2FeO4)�����Ժӡ����ĵ�ˮ�����dz�������ˮ�����¼��������ж�Na2FeO4����������ˮ�����;���������ԭ�������ȷ����( )
A.Na2FeO4����Һ����ǿ���ԣ�������ɱ��
B.Na2FeO4�Ļ�ԭ������Fe3+����ˮ������Fe(OH)3����ʹˮ�����������۳���
C.Na2FeO4�Ļ�ԭ������Fe2+����ˮ��ΪFe(OH)2����ʹˮ������������?����
D.Na2FeO4��FeΪ+6�ۣ�����ǿ�����ԣ�������ɱ��
����(��)��������һ��ˮ�����������Ʊ������д�������������(ʪ��)���÷����ڼ�����Һ�У����ô������������������Σ���д������ƽ��һ���ӷ�Ӧ����ʽ��__________________��
(4)�ҹ��Ϸ�ijЩ�����ľ�ˮȡ�����ú��ɳ���������������غ�ɫ��״���������ڸף�ˮ�渡��һ�㡰��Ƥ�������ؾ����ü������ķ����������پ�ˮ���ú�ˮ�渡�ŵ�һ�㡰��Ƥ������Ҫ�ɷ���_________(д��ѧʽ)�����û�ѧ��������þ�ˮ�IJ�����__________________�����ӷ���ʽ��__________________�����û�ѧ�������������������ˮ�IJ�����__________________�����ӷ���ʽ��__________________����AlCl3��6H2O(����AC)�ͣ�Al2(OH)nCl6-n��m(����BAC)��������Ϊˮ����������Ƕ�ˮ��pH�ı��Ӱ���Ƿ�һ����_________��ԭ����ʲô��__________________��
(5)X����Ҳ����������ˮ�����������������Ч�ʣ��ְ�ȫ������ζ�������ã�����������Ȼ���д��ڣ��Ե��������𱣻�ɡ���á�X�Ļ�ѧʽ��_________��X��ʹʪ��ĵ��۵⻯����ֽ��������д���÷�Ӧ�Ļ�ѧ����ʽ��__________________���÷�Ӧ��������������ͻ�ԭ��������ʵ���֮��Ϊ_________��
(6)ClO2����Ϊ�����Ĵ�������ˮɱ�����������Ч������Ⱦ�����㷺ʹ�á��ҹ����������Ƽ�����˾���õ�ⷨ��������ClO2���䷴Ӧԭ��Ϊ![]() +4 H+
+4 H+![]() 4ClO2��+O2��+2H2O���ӷ�Ӧԭ������Ӧ�����������½��У��ܷ������������Һ�����ԣ�
4ClO2��+O2��+2H2O���ӷ�Ӧԭ������Ӧ�����������½��У��ܷ������������Һ�����ԣ�
��________(��ܡ����ܡ�)��������________________________________��
����:�������ڲ���ʽ���⣬����ѧ�γ������й���������������֪ʶ������һ��������漰�����ݱȽϹ㣬��ͬѧ��ֻҪ�������Ԫ�ػ�����֪ʶ���й����ۼ��ܵý⡣
��:
(1)Cl2+H2O====H++Cl-+HClO
2Fe2++Cl2====2Fe3++2Cl-
(2)CH4+Cl2![]() CH3Cl+HCl
CH3Cl+HCl
CH3Cl+Cl2![]() CH2Cl2+HCl
CH2Cl2+HCl
CH2Cl2+Cl2![]() CHCl3+HCl ȡ�� ����
CHCl3+HCl ȡ�� ����
(3)BD 2Fe3++3ClO-+10 OH-====![]() +3Cl-+5H2O
+3Cl-+5H2O
(4)��Fe(OH)3 ��ȡ����ˮ�����Թ��У��μ�����NH4SCN��KSCN��Һ������Һ�ʺ�ɫ����Ϊ�þ�ˮ����Fe3+ Fe3++3SCN-====Fe(SCN)3 ��������������ˮ�к������϶��![]() ������ˮ���м�������BaCl2��Һ�����������ɫ���ǣ���Ϊ������������ˮ Ba2++
������ˮ���м�������BaCl2��Һ�����������ɫ���ǣ���Ϊ������������ˮ Ba2++![]() ====BaSO4��(���������������Ϊʵ���������ԣ�������Ѵ�) �ܲ�һ�� BAC�ɿ���AC��ˮ��ˮ������е��м�����˶�ˮ��pH�ı�û��AC��
====BaSO4��(���������������Ϊʵ���������ԣ�������Ѵ�) �ܲ�һ�� BAC�ɿ���AC��ˮ��ˮ������е��м�����˶�ˮ��pH�ı�û��AC��
(5)O3 O3+2KI+H2O====O2+I2+2KOH 1��1
(6)���� �����������£�Cl-�ܱ�![]() ������Cl2����������Ƶ�ClO2��
������Cl2����������Ƶ�ClO2��![]() +5Cl-+6 H+====3Cl2��+3H2O
+5Cl-+6 H+====3Cl2��+3H2O

 �п�������㾫��ϵ�д�
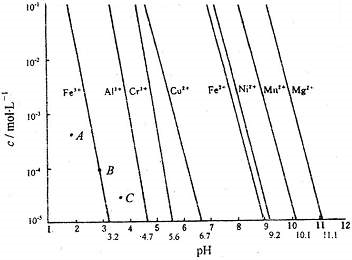
�п�������㾫��ϵ�д���10�֣�����ˮ��ȫ״�������ཡ���������й�ϵ���ؽ������ӶԺ�������������������Ⱦ���������Ǵ������ؽ���������ˮ���õķ�����
ij������ˮ��pH��2.0���ѣ�1.0 g��mL-1���к�Ag+��Pb2+�� �ؽ������ӣ���Ũ�ȸ�ԼΪ0.01mol��L-1���ŷ�ǰ���ó�������ȥ���������ӣ������й��������£�
| ���ܵ���� | AgCl | AgI | AgOH | Ag2S | PbI2 | Pb(OH)2 | PbS |
| Ksp | 1.8��10-10 | 8.3��10-17 | 5.6��10-18 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |
��1������Ϊ����ˮ��Ͷ ������ĸ��ţ�������Ч����á�
A��NaOH B��Na2S C��KI D��Ca(OH)2
��2���������ʯ�Ҵ���������ˮ��ʹ��Һ��pH��8.0��������ķ�ˮ��c(Pb2+)�� ��
��3�������ʳ�δ�����ֻ�������ӵķ�ˮ����ô�����ķ�ˮ��NaCl����������Ϊ0.117%��������Ҫ���ŷű�Ϊc(Ag+)����1.0��10-18mol��L-1����ù���������ķ�ˮ�Ƿ�����ŷű� ������ǡ���д��������̡�