��Ŀ����
Ϊ��֤��ͭ��ϡ���ᷴӦ������������һ��������Ϊ��ɫ��ijУѧ��ʵ��С�������һ������ͼ��ʾ������װ�ú̶�װ�þ�����ȥ����ʵ��װ�ã����У�A����һ���ý���˿�̶��ĸ���ܣ���װ��״̼��ƹ��壻EΪһ���յ�������ƿ��F�����ڹ��������˫������������������⣺��1��Ϊ����E��ƿ�о������ռ���NO���Թ۲�����ɫ����ʵ��ʱ�������Ƚ�E�еĿ������ߡ����߿����ľ���IJ���Ϊ________________________________________��
��2�����֤��E���ռ���������NO������H2______________________________��
��3��A��ͭ˿��ϡ���ᷴӦ����װ��A�ȣ���������ɫ��������ӷ���ʽΪ__________
______________________________��
��4������ʵ������У�C�Թ����ȳ��ֻ��Ǻ������ԭ����_______________________������������Һ��������__________________________________��
��5��������Ϊ��ʵ�鲻��֮����E�еĽ�������̫�������飺��E�еĿ�������ʱ�����Eװ���н������̳ܶ������ܳ���Ȼ�����ʵ�飬��Eװ�����ռ�NOʱ�������ܳ��������̡ܶ���������__________________________________��
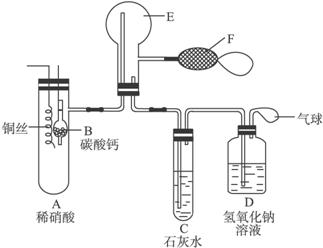
��1����ʢ��̼��Ƶĸ��������Aװ���е�ϡ��������߷�����Ӧ����CO2������CO2���ܶȱȿ������ܰ�װ��ϵͳ�ڵĿ����ų���
��2����˫����������E�д�����������E������������ɫ��Ϊ����ɫ��˵��E���ռ�����������NO������H2��
��3��ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ��3Cu+8H++2![]() ====3Cu2++2NO��+4H2O��
====3Cu2++2NO��+4H2O��
��4��C�Թ����ȳ��ֻ�������ΪCO2��ʯ��ˮ��Ӧ��Ca(OH)2+CO2====H2O+CaCO3����Ȼ���ֱ���壬����ΪNO2��Cװ���е�ˮ��Ӧ����HNO3����CaCO3�����ܽ⡣����һ���ܵ�ԭ����ͨ���CO2��������CaCO3�����ܽ⣬����������ˮ��Ca(HCO3)2��Һ�����������ɵ������ж�����Ҫ����β��������NaOH��Һ�����þ�������β������ֹ��Ⱦ������
��5�����ų�Eװ���еĿ���ʱ����ΪCO2���ܶȱȿ�����Eװ���н������̳ܶ������ܳ����������ڰ�E�п����ų�����Eװ�����ռ�NO����ʱ����ΪNO���ܶȱ�CO2���ܶ�С��Eװ���н������ܳ��������̣ܶ������ڰ�Eװ���е�CO2�ų���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�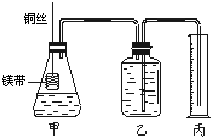 ��ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ
��ͭ��ϡ���ᷴӦ�����ӷ���ʽΪ