��Ŀ����
20��${\;}_{92}^{235}$U����Ҫ�ĺ˹�ҵԭ�ϣ�����Ȼ��ķ�Ⱥܵͣ�${\;}_{92}^{235}$U��Ũ��һֱΪ��������ע�������й�${\;}_{92}^{235}$U˵����ȷ���ǣ�������| A�� | ${\;}_{92}^{235}$U ԭ�Ӻ��к���92������ | |
| B�� | ${\;}_{92}^{235}$U ԭ�Ӻ�����143������ | |
| C�� | ${\;}_{92}^{235}$U ��������Ϊ92 | |
| D�� | ${\;}_{92}^{235}$U ��${\;}_{92}^{238}$U ��Ϊͬλ�� |
���� 92235U��������Ϊ92��������Ϊ235�����������ں����������������=������+��������ͬλ�صķ���������ԭ�ӣ�
��� �⣺A��92235U��������Ϊ235-92=143����A����
B��92235U��������Ϊ92�����������ں��������������������Ϊ92����B����
C��92235U��������Ϊ92��������Ϊ235����C����
D��92235U��92238U����������ͬ������������ͬ����Ӧ��Ϊͬλ�أ���D��ȷ��
��ѡD��
���� ���⿼��ԭ�ӵĹ��ɼ�ԭ���е����Ĺ�ϵ����ȷԭ�ӹ����в�ͬλ�õ����ֱ�ʾ�����塢���������ں����������������=������+��������ͬλ�غ�ͬ��������ĸ���ɽ��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
10����֪A��g��+B��g��?C��g��+D��g����Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���±���������
830��ʱ����һ��2L���ܱ������г���0.2mol��A��0.8mol��B����Ӧ��ʼ4s��A��ƽ����Ӧ����v��A��=0.005mol/��L��s��������˵����ȷ���ǣ�������
| �¶�/�� | 700 | 800 | 830 | 1000 | 1200 |
| ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
| A�� | 4sʱc��B��Ϊ0.76mol/L | |
| B�� | 830���ƽ��ʱ��A��ת����Ϊ80% | |
| C�� | ��Ӧ��ƽ��������¶ȣ�ƽ�������ƶ� | |
| D�� | 1200��ʱ��ӦC��g��+D��g��?A��g��+B��g����ƽ�ⳣ����ֵΪ0.4 |
15��ij��ѧ�о���ѧϰС����CO��ԭFe2O3������ʵ��������ô����������ɵĺ�ɫ��ĩX����̽����
[̽��Ŀ��]������ɫ��ĩX����ɣ����������ʵ�飮
[��������]
��CO��ԭFe2O3��ʵ�������¶Ȳ�ͬ�����Ȳ���ʱ������Fe3O4��Ҳ�ܱ�����������
��Fe3O4+8H+=2Fe3++Fe2++4H2O
��Fe+4HNO3��ϡ��=Fe��NO3��3+NO��+2H2O
��3Fe3O4+28HNO3��ϡ��=9Fe��NO3��3+NO��+14H2O
[ʵ��̽��]
I�����Լ���
��1��ʵ��۷����ķ�Ӧ�����ӷ���ʽΪFe+Cu2+=Fe2++Cu��
��2������ʵ��˵����ɫ��ĩX�к���Fe3O4��Fe�Ļ���
II�������ⶨ
������ͼ��ʾ��ʵ�鷽������ʵ�鲢��¼���ݣ�
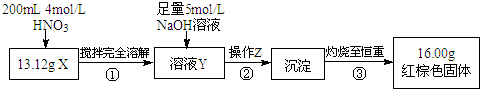
��1������Z�������ǹ��ˣ�
��2��ͨ���������ݣ��ó�13.12g��ɫ��ĩX�и��ɷֵ����ʵ���ΪFe��0.11mol��Fe3O4��0.03mol��
��3������ҺY�������Ϊ200mL������ҺY��c��Fe3+��=1mol/L��
[̽��Ŀ��]������ɫ��ĩX����ɣ����������ʵ�飮
[��������]
��CO��ԭFe2O3��ʵ�������¶Ȳ�ͬ�����Ȳ���ʱ������Fe3O4��Ҳ�ܱ�����������
��Fe3O4+8H+=2Fe3++Fe2++4H2O
��Fe+4HNO3��ϡ��=Fe��NO3��3+NO��+2H2O
��3Fe3O4+28HNO3��ϡ��=9Fe��NO3��3+NO��+14H2O
[ʵ��̽��]
I�����Լ���
| ��� | ʵ����� | ʵ������ |
| �� | ȡ������ɫ��ĩX�����Թ�1�У�ע��Ũ���ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ�ʻ���ɫ�������ݲ��� |
| �� | ���Թ�1�еμӼ���KSCN��Һ���� | ��Һ����Ѫ��ɫ |
| �� | ��ȡ������ɫ��ĩX�����Թ�2�У�ע����������ͭ��Һ�������� | �м�������ɫ�������������н϶��ɫ����δ�ܽ� |
��2������ʵ��˵����ɫ��ĩX�к���Fe3O4��Fe�Ļ���
II�������ⶨ
������ͼ��ʾ��ʵ�鷽������ʵ�鲢��¼���ݣ�

��1������Z�������ǹ��ˣ�
��2��ͨ���������ݣ��ó�13.12g��ɫ��ĩX�и��ɷֵ����ʵ���ΪFe��0.11mol��Fe3O4��0.03mol��
��3������ҺY�������Ϊ200mL������ҺY��c��Fe3+��=1mol/L��
5������ƿ�ϱ��е��Ǣ��¶� ��Ũ�� �����ʵ��� �ܹ�� �ݿ̶��� ����ʽ���ʽ��������
| A�� | �٢ۢ� | B�� | �ۢݢ� | C�� | �٢ܢ� | D�� | �ڢۢ� |
9����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | 2.0gH218O��D2O�Ļ����������������ΪNA | |
| B�� | 1mol Na2O2 �����к���������Ϊ4 NA | |
| C�� | ��״���£�22.4 LSO3����NA������ | |
| D�� | 50ml 12mol/L����������MnO2���ȣ�ת�Ƶĵ�����Ϊ0.3NA |
10�������йض����ԭ�ӵ������У���ȷ���ǣ�������
| A�� | ��һ�������ԭ���У�������������������ͬ�ĵ��� | |
| B�� | ��һ�������ԭ���У��������������˶�״̬��ȫ��ͬ�ĵ��� | |
| C�� | ��һ�������ԭ���У�N���ϵĵ��������϶���M���ϵĵ��������� | |
| D�� | ij�������ԭ�ӵ�3p�ܼ��Ͻ����������ӣ����DZ�Ȼ����״̬��ͬ |
 ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ������ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩 �ߵζ���
ʵ�����ù���NaOH����0.5mol/L��NaOH��Һ500mL����������������Ʒ�У����ձ� ��100mL��Ͳ ������ƿ ��ҩ�� �ݲ����� ��������ƽ�������룩 �ߵζ���