��Ŀ����
3��ͨ������£�CO��һ����ɫ����ζ���ж������壬������ˮ�����ᡢ�����Һ������Ӧ���ƾ���ƿ���������Դ����ȷ������ͼ��ʾ��װ�ý���ʵ�飬������֤ij�������ijɷ���CO2��CO��ÿ��װ������һ�Σ�����ش��������⣺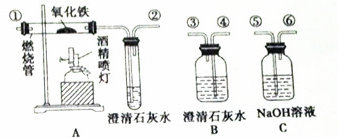
��1������װ�õ��ܿڵ�˳���������ܢۢݢޢ٢ڡ�β����������ܽӿڴ��ţ���
��2��֤��ԭ���������CO2���ڵ�ʵ��������B��������A�в������ڷ�ĩ�ɺ��ڣ��Թ��ڳ���ʯ��ˮ����ǣ�֤��CO���ڵ��йط�Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��Ca��OH��2+CO2=CaCO3��+H2O
��3����ͬѧ�������BӦ��ʹ��һ�Σ�����Ϊ�е������У���С���û�С�������������������B��C��A����˵��������̼��C���Ѿ���������
��4����ʵ��β�������ķ������ڵ��ܢۺ����һ��ȼ�ŵľƾ��ƣ����������ռ�����
���� ��1��װ��A���ڼ���CO�Ĵ��ڣ��ѻ��������CO2��ȫ����������ͨ��Aװ�ã����������ڷ�ĩ�ɺ�ɫ���ɫͬʱ�Թ��еij���ʯ�ұ���ǣ�˵����������к�CO��
��2��װ��B���ڼ����������к�CO2���������ͨ��װ��B������ʯ��ˮ����ǣ�˵����������к���CO2��
��3����Ҫ���ó���ʯ��ˮ֤��ԭ��������еĶ�����̼�Ѿ��������������CO�ļ��飻
��4��һ�������ж���β���к��е�һ����̼��Ҫ���е�ȼ���ռ���
��� �⣺��1���������ѡͨ��װ��B����CO2�����Ƿ���ڣ�����Ӧ�ɳ��ܢ�ͨ��Ӷ̹ܢ��ų�Bװ�ã�Ȼ��ͨ��װ��C��������������е�����CO2���ɢ�ͨ��Ӣ��ų�������������ͨ��װ��A������CO����Ĵ��ڣ������ɢٽ���Ӣ��ų����������ӵ��ܿڵ�˳��Ϊ���ܢۢݢޢ٢ڣ�
�ʴ�Ϊ���ܢۢݢޢ٢ڣ�
��2����ֻ��CO��B�г���ʯ��ˮ������ǣ�A�в������ڷ�ĩ�ɺ��ڣ�˵������������ԭ�������Թ��ڳ���ʯ��ˮ����ǣ�˵�����ɶ�����̼���壬���������ۺϣ�˵�����������ֻ����CO���壬��Ӧ�Ļ�ѧ����ʽ�У�Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��Ca��OH��2+CO2=CaCO3��+H2O��
�ʴ�Ϊ��B��������A�в������ڷ�ĩ�ɺ��ڣ��Թ��ڳ���ʯ��ˮ����ǣ�Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��Ca��OH��2+CO2=CaCO3��+H2O��
��3����������B��C��A����˵��������̼��C���Ѿ���������Ӧ����ʹ��һ������B֤��������̼�����Ѿ�������
�ʴ�Ϊ���ǣ�����B��C��A����˵��������̼��C���Ѿ���������
��4��β���к����ж���CO���壬��Ҫ����β������������Ϊ�����ڵ��ܢۺ����һ��ȼ�ŵľƾ��ƻ��������ռ���
�ʴ�Ϊ���ڵ��ܢۺ����һ��ȼ�ŵľƾ��ƣ����������ռ�����
���� ���⿼���˳�����������ʼ����鷽������ƣ���Ŀ�Ѷ��еȣ���ȷ������������ʼ����鷽��Ϊ���ؼ���������ؿ���ѧ���ķ�����������ѧʵ��������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 150mL 2 mol•L-1 KCl��Һ | B�� | 75mL 1.5 mol•L-1 MgCl2��Һ | ||
| C�� | 100mL 1 mol•L-1 NaCl��Һ | D�� | 25mL 2 mol•L-1 AlCl3��Һ |
| A�� | ��������ԭ��Ӧ�У��϶���һ��Ԫ�ر���������һ��Ԫ�ر���ԭ | |
| B�� | ������ԭ�ı�������Ԫ�ػ��ϼ۵����� | |
| C�� | ͬһ������ԭ��Ӧ��������������ԭ��Ӧ����ԭ������������Ӧ | |
| D�� | ʧ���ӵķ�Ӧ���ڷ�Ӧ������������������ԭ��Ӧ |
| A�� | 4L 0.5mol•L-1NaCl��Һ | B�� | 1L 0.3mol•L-1Na2SO4��Һ | ||
| C�� | 2L 0.15mol•L-1Na2CO3��Һ | D�� | 0.8L 0.4mol•L-1NaOH��Һ |
| A�� | ������Ʒ����һ��ʱ���pH������ | |
| B�� | �����¶ȵ����ߣ���ˮpH���� | |
| C�� | ������ˮ������һ��ʱ���pH��С | |
| D�� | ����������Һ�����ڿ����У�pH���� |
| A�� | ����-20���ɻ���ʹ�õ�̼��ά��һ�����͵��л��߷��Ӳ��� | |
| B�� | ���������������ڽ��ػ����������������ָ��£������������ǽ������� | |
| C�� | ����������Ƕп�飬п���������Է����屻��ʴ | |
| D�� | �����������ɸ��ͣ��������������Ƶã����������� |