��Ŀ����
6����ͼ��Ԫ�����ڱ���һ���֣�����բ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺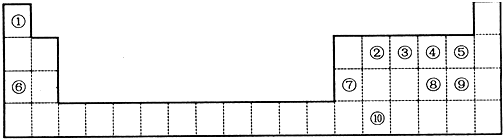
��1��Ԫ�آ��γɵĵ��ʵĵ���ʽΪ
 ��Ԫ�آ��γɵ����������ĽṹʽΪO=C=O��
��Ԫ�آ��γɵ����������ĽṹʽΪO=C=O����2���ޡ�����Ԫ�ص�����������ˮ����֮�䷴Ӧ������ʽΪAl��OH��3+OH-=AlO2-+2H2O��
��3���ڡ��ۡ��ܡ���Ԫ���γɵ��⻯���У����ȶ�����ǿ����HF���ѧʽ�����е���ߵ���H2O�ѧʽ����
��4���ࡢ��Ԫ�ص�����������ˮ��������ǿ���Ƚ�HClO4��H2SO4���ѧʽ����
��5���ޡ�����Ԫ����ɵĻ����������Ӽ����ѧ������
���� ����Ԫ�������ڱ��е����λ�ÿ�֪������H������C������N������O������F������Na������Al������S������Cl������Ge��
��1��Ԫ�آ��γɵĵ���Ϊ�����������д��ڵ���������Ԫ�آ��γɵ����������Ϊ������̼��������̼�����к�������̼��˫����Ϊ���ۻ����
��2������Na������Al�����ߵ����������ֱ�Ϊ�������ƺ�������������������������������Ӧ����ƫ�����ƺ�ˮ��
��3���ǽ�����Խǿ����Ӧ��̬�⻯��Խ�ȶ���
��4������Ԫ������������ˮ�������Եݱ���ɷ�����
��5��������Ԫ���γɵĻ����Ϊ���ƣ��������ӻ�����������Ӽ���
��� �⣺����Ԫ�������ڱ��е����λ�ÿ�֪������H������C������N������O������F������Na������Al������S������Cl������Ge��
��1��Ԫ�آ��γɵĵ���Ϊ�����������ĵ���ʽΪ�� ��
��
Ԫ�آ��γɵ����������Ϊ������̼��������̼Ϊ���ۻ����������к�������̼��˫�����ṹʽΪ��O=C=O��
�ʴ�Ϊ�� �� O=C=O��
�� O=C=O��
��2������Na������Al���������ƺ�����������Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��3������C������N������O������F������ͬ���ڴ�����Ԫ���⻯���ȶ�������ǿ��֪���ȶ�����ǿ����HF���е���ߵ�Ӧ����H2O����Ϊ���⼸��Ԫ�ص��⻯����ֻ��H2O�ڳ�������Һ�壬���������壨Ҳ�ɴ�������ͣ�����ˮ�ķе���ߣ�
�ʴ�Ϊ��HF��H2O��
��4���ࡢ��ֱ���S��Cl������ͬ��������������ˮ�����������������ǿ��֪������Ϊ��HClO4��H2SO4��
�ʴ�Ϊ��HClO4��H2SO4��
��5���ޡ�����Ԫ�طֱ�ΪNa��S�������γɵĻ�����Ϊ���ƣ�����Ϊ���ӻ�������еĻ�ѧ��Ϊ���Ӽ���
�ʴ�Ϊ�����Ӽ���
���� ���⿼����Ԫ�����ڱ��Ľṹ��Ԫ��λ�á����ɱ仯��Ӧ�ã�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ���ȷԪ�����ڱ��ṹ��Ԫ������������Ϊ���ؼ�������������ѧ���ķ������������Ӧ�û���֪ʶ��������

 ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�| A�� | ����ӵ���ѳ�Ϊ�ʼDZ����ԡ��ƶ��绰�ȵ��ĵ�����������Դ | |
| B�� | ����ȼ�ϵ�ؿɽ���ѧ��Ӧ������ֱ��ת��Ϊ���� | |
| C�� | Ǧ���طŵ�ʱǦ�ڸ����������ɶ�����Ǧ | |
| D�� | п�̸ɵ�ع���һ��ʱ���̼����ϸ |
��Ư�ۡ�ˮ���������ȼ����ǻ���� ����Ȼ����Һ��ʯ��������Ҫ�ɷֶ��Ǽ���
��Fe2O3�׳����죬���������Ϳ�� ��ʳ�Ρ���������Ƕ��ǵ����
��ʯ�͵��ѽ⡢ú�����������ϻ�����ˮ��þ�Ĺ��̶�������ѧ�仯
��������������������������ֹ��������ʴ��
| A�� | �ڢܢ� | B�� | �٢ۢ� | C�� | �٢ڢ� | D�� | �ۢܢ� |
| A�� | 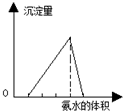 ͼ�ɱ�ʾ���ữ��AlCl3��Һ����μ���ϡ��ˮ���������백ˮ����Ĺ�ϵ | |
| B�� | 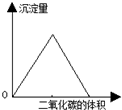 ͼ�пɱ�ʾ�����ʯ��ˮ��ͨ�������̼���壬�������������̼����Ĺ�ϵ | |
| C�� |  ͼ�пɱ�ʾ����������Һ��ͨ�����⣬����������������Ĺ�ϵ | |
| D�� | 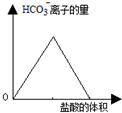 ͼ�пɱ�ʾ��̼������Һ����μ���ϡ���ᣬHCO3-���ӵ�������������Ĺ�ϵ |
| A�� | 1mol���� | B�� | 1mol S | ||
| C�� | ��1molHNO3��ϡ���� | D�� | ��1molCuSO4������ͭ��Һ |
| A�� | 0.1mol•L-1 FeSO4��Һ�У�K+��NH4+��MnO4-��ClO- | |
| B�� | ����������Һ�У�Fe3+��Mg2+��SO42-��Br- | |
| C�� | c��H+��=$\sqrt{{K}_{W}}$����Һ�У�K+��Al3+��Cl-��SO42- | |
| D�� | ʹ��̪���ɫ����Һ��Na+��NH2CH2COOH��I-��Ba2+ |
| A�� | ԭ�Ӱ뾶��Y��Z��R��T | |
| B�� | XR2��WR2����������R�Ļ��ϼ���ͬ | |
| C�� | ����������Ӧˮ����ļ��ԣ�X��Z | |
| D�� | ��̬�⻯����ȶ��ԣ�W��R��T |