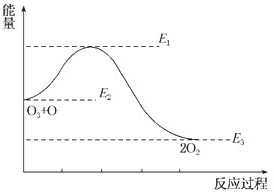
��Ŀ����
��25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ�����б�ʾ�״�ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ�ǣ�������
A��CH3OH��l��+
| ||
| B��2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��l����H=-1452kJ?mol-1 | ||
C��CH3OH��l��+
| ||
D��CH3OH��l��+
|
A����Ӧ�Ƿ��ȷ�Ӧ����A����
B���Ȼ�ѧ����ʽ��2mol�״�ȼ�շų�����������B����
C��������ľۼ�״̬δ��ע����C����
D��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����725.8KJ��ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+
O2��g���TCO2��g��+2H2O��l����H=-725.8 kJ?mol-1����D��ȷ��
��ѡD��
B���Ȼ�ѧ����ʽ��2mol�״�ȼ�շų�����������B����
C��������ľۼ�״̬δ��ע����C����
D��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����725.8KJ��ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH��l��+
| 3 |
| 2 |
��ѡD��

��ϰ��ϵ�д�
�����Ŀ