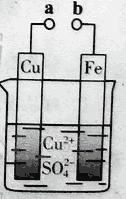
题目内容
【题目】据报道,化合物M对番茄灰霉菌有较好的抑菌活性,其合成路线如下图所示。


完成下列填空:
(1)写出反应类型。
反应③___________ 反应④__________
(2)写出结构简式。
A______________ E_______________________
(3)写出反应②的化学方程式________________
(4)B的含苯环结构的同分异构体中,有一类能发生碱性水解,写出检验这类同分异构体中的官能团(酚羟基除外)的试剂及出现的现象。
试剂(酚酞除外)___________ 现象________________________
(5)写出两种C的含苯环结构且只含4种不同化学环境氢原子的同分异构体的结构简式。_____________
(6)反应①、反应②的先后次序不能颠倒,解释原因。___________________
【答案】 还原 取代 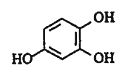
 银氨溶液(新制氢氧化铜悬浊液) 有银镜出现(有砖红色沉淀产生)
银氨溶液(新制氢氧化铜悬浊液) 有银镜出现(有砖红色沉淀产生) 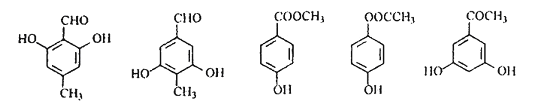 B中有酚羟基,若硝化,会被硝酸氧化而降低M的产率
B中有酚羟基,若硝化,会被硝酸氧化而降低M的产率
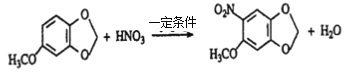
【解析】(1)根据合成线路中的反应条件和已知信息,反应③是将—NO2转化为—NH2,即是去氧加氢的反应,属于还原反应;根据M的结构简式,反应④是—COOH与—NH2反应生成—CONH—(肽键),属于取代反应;
(2)根据M的结构简式和信息1可知,A中有三个羟基,处于两邻一间的位置上,所以A的结构简式为![]() , 再结合反应④可确定E的结构简式为
, 再结合反应④可确定E的结构简式为![]() ;
;
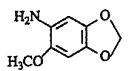
(3)通过A和E的结构简式,可推知B为![]() ,C为
,C为![]() D为
D为![]() ,则反应②的化学方程式
,则反应②的化学方程式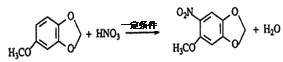
(4)B的结构简式为![]() ,含苯环结构的同分异构体中,能发生碱性水解的为酚羟基与甲酸形成的甲酸酯,甲酸酯具有醛基的性质,可用银氨溶液或新制氢氧化铜悬浊液检验;现象是有银镜出现或有砖红色沉淀产生;
,含苯环结构的同分异构体中,能发生碱性水解的为酚羟基与甲酸形成的甲酸酯,甲酸酯具有醛基的性质,可用银氨溶液或新制氢氧化铜悬浊液检验;现象是有银镜出现或有砖红色沉淀产生;
(5)C的结构简式为![]() ,其同分异构体中除含有苯环结构外,还一定有一个双键,因此可以构成—CHO、或—COOH或—COO—或
,其同分异构体中除含有苯环结构外,还一定有一个双键,因此可以构成—CHO、或—COOH或—COO—或![]() 等,只含4种不同化学环境氢原子的同分异构体的结构简式有:
等,只含4种不同化学环境氢原子的同分异构体的结构简式有: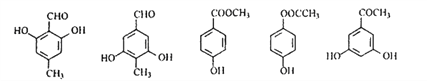
(6) B的结构(![]() )中含有酚羟基,很容易被氧化,若反应①、②颠倒,先发生硝化反应时硝酸可氧化酚羟基,降低M的产率。
)中含有酚羟基,很容易被氧化,若反应①、②颠倒,先发生硝化反应时硝酸可氧化酚羟基,降低M的产率。

 手拉手全优练考卷系列答案
手拉手全优练考卷系列答案【题目】下列物质提纯的方案错误的是
选项 | 被提纯的物质 | 杂质 | 除杂试剂 | 除杂方法 |
A | CO | CO2 | NaOH溶液,浓H2SO4 | 洗气 |
B | CO2 | HCl | NaOH溶液 | 过滤 |
C | Cl2 | HCl | 饱和食盐水,浓H2SO4 | 洗气 |
D | Na2CO3固体 | NaHCO3固体 | —— | 加热 |
A.AB.BC.CD.D
【题目】学习化学有利于我们认识饮食与健康的关系,养成良好的饮食习惯.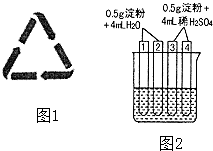
(1)油脂被摄人人体后,在酶的作用下水解为(写名称,下同)和 , 进而被氧化生成并提供能量,或作为合成人体所需其他物质的原料.
(2)发育出现障碍,患营养缺乏症,这主要是由于摄取(填“蛋白质”、“脂肪”或“糖类”)不足引起的.下列食物中富含该物质的是 (填字母).
A菠菜 B花生油 C瘦肉 D西瓜
(3)某火腿制品的包装上印有相关配料:精选瘦肉、白糖、淀粉、亚硝酸钠等.火腿中属于防腐剂的是 , 不可长期或大量进食腌制肉类食品的原因是:其包装袋上常可看到如图1所示的图标,它的含义是 , 此包装袋材料是聚乙烯塑料,它的单体是(填结构简式).
(4)如图2所示4支试管同时水浴加热4min.为检验其中淀粉的水解程度,某同学的实验操
作与现象记录如下:
试管 | 操作 | 现象 |
① | 加入碘水 | 溶液变成蓝色 |
② | 加入银氨溶液,水浴加热 | 未出现银镜 |
③ | 加入碘水 | 溶液变成蓝色 |
④ | 加入银氨溶液,水浴加热 | 未出现银镜 |
①结合试管1、2中的现象,得出的结论是:这两支试管中淀粉水解(填“没有”、“部分”或“全部”),淀粉 (填“有”或“没有”)还原性.
②结合试管3、4中的现象,(填“能”或“不能”)得出“这两支试管中淀粉没有水解”的结论,理由是 .