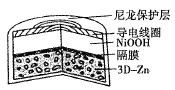
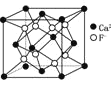
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ≈πΦΑΤδΜ·ΚœΈο‘Ύ–¬≤ΡΝœΓΔΙΛ≈©“Β…ζ≤ζΒ»ΖΫΟφ”ΟΆΨΚήΙψΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)–¥≥ω”κB‘ΣΥΊΆ§÷ςΉεΒΡGa‘ΣΥΊΒΡΜυΧ§‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΘΚ________ΓΘ
(2)ΝΔΖΫΒΣΜ·≈π(BN)Ω…άϊ”Ο»ΥΙΛΖΫΖ®‘ΎΗΏΈ¬ΗΏ―ΙΧθΦΰœ¬Κœ≥…Θ§ τ”Ύ≥§”≤≤ΡΝœΓΘΆ§ τ‘≠Ή”ΨßΧεΒΡΒΣΜ·≈π±»ΨßΧεΙηΨΏ”–ΗϋΗΏΒΡ”≤Ε»ΚΆΡΆ»»–‘ΒΡ‘≠“ρ «__________ΓΘ
(3)BF3Ζ÷Ή”÷–÷––Ρ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά «____ΓΘ”÷÷Σ»τ”–dΙλΒά≤Έ”κ‘”Μ·Θ§Ρή¥σ¥σΧαΗΏ÷––Ρ‘≠Ή”ΒΡ≥…ΦϋΡήΝΠΘ§Ζ÷ΈωBF3ΓΔSiF4Υ°ΫβΒΡ≤ζΈο÷–Θ§ ≥ΐΝΥœύ”ΠΒΡΥαΆβΘ§«Α’Ώ…ζ≥…BF4-ΕχΚσ’Ώ…ζ≥…SiF62-ΒΡ‘≠“ρΘΚ_______________ΓΘ
(4)NaBH4±Μ»œΈΣ «”–ΜζΜ·―ß÷–ΒΡΓΑΆρΡήΜΙ‘≠ΦΝΓ±Θ§NaBH4ΒΡΒγΉ” ΫΈΣ_________Θ§Τδ÷–»ΐ÷÷‘ΣΥΊΒΡΒγΗΚ–‘”…¥σΒΫ–ΓΒΡΥ≥–ρ «_______________ΓΘ
(5)Ή‘»ΜΫγ÷–Κ§≈π‘ΣΥΊΒΡΡΤ―Έ «“Μ÷÷Χλ»ΜΩσ≤ΊΘ§ΤδΜ·―ß Ϋ–¥Ής Na2B4O710H2OΘ§ ΒΦ …œΥϋΒΡΫαΙΙΒΞ‘Σ «”…ΝΫΗωH3BO3ΚΆΝΫΗωB(OH)4]-(ΚœΕχ≥…ΒΡΥΪΝυ‘ΣΜΖΘ§”ΠΗΟ–¥≥… Na2[B4O5(OH)4]8H2OΘ§ΤδΫαΙΙ»γΆΦΥυ ΨΘ§ΥϋΒΡ“θάκΉ”Ω…–Έ≥…Ν¥Ή¥ΫαΙΙΘ§‘ρΗΟΨßΧε÷–≤Μ¥φ‘ΎΒΡΉς”ΟΝΠ «______________(ΧνΉ÷ΡΗ)ΓΘ
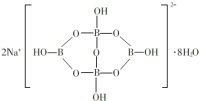
AΘ°άκΉ”Φϋ BΘ°Ι≤ΦέΦϋ CΘ°«βΦϋ DΘ°Ϋπ τΦϋ EΘ°ΖΕΒ¬ΜΣΝΠ
(6)ΝΉΜ·≈π(BP)Ω…ΉςΈΣΫπ τ±μΟφΒΡ±ΘΜΛ±ΓΡΛΘ§ΤδΨßΑϊ»γΆΦΥυ ΨΘ§‘ΎBPΨßΑϊ÷–P’ΦΨίΒΡ «≈π‘≠Ή”Ε―ΜΐΒΡ_____(ΧνΓΑΝΔΖΫΧεΓ±ΓΑ’ΐΥΡΟφΧεΓ±ΜρΓΑ’ΐΑΥΟφΧεΓ±)Ω’œΕΓΘΫ®ΝΔ»γΆΦΥυ ΨΉχ±ξœΒΘ§Ω…ΒΟΨßΑϊ÷–AΓΔC¥Π‘≠Ή”ΒΡΖ÷ ΐΉχ±ξΘ§‘ρN¥ΠΒΡP‘≠Ή”Ζ÷ ΐΉχ±ξΈΣ______ΓΘ»τΨßΑϊ÷–≈π‘≠Ή”ΚΆΝΉ‘≠Ή”÷°ΦδΒΡΉνΫϋΚΥΦδΨύΈΣa pmΘ§‘ρΨßΑϊ±Ώ≥ΛΈΣ____________cmΓΘ
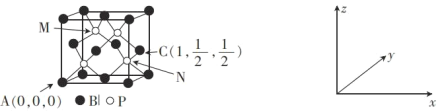
ΓΨ¥πΑΗΓΩ1s22s22p63s23p63d104s24p1(Μρ[Ar]3d104s24p1) N‘≠Ή”ΚΆB‘≠Ή”ΒΡΑκΨΕ±»Ιη‘≠Ή”–ΓΘ§B-NΦϋ≥Λ±»Si-SiΕΧΘ§ΦϋΡήB-NΘΨSi-SiΦϋ sp2 B‘≠Ή”ΉνΆβΒγΉ”≤ψΈΣL≤ψΘ§ΈόdΙλΒάΘ§ΕχSi‘≠Ή”ΉνΆβ≤ψΈΣM≤ψΘ§”–dΙλΒάΘ§Ω…≤Έ”κ‘”Μ·Θ§ ΙSi≈δΈΜ ΐ‘ωΦ”÷Ν6 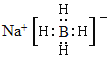 HΘΨBΘΨNa D ’ΐΥΡΟφΧε (
HΘΨBΘΨNa D ’ΐΥΡΟφΧε (![]() Θ§
Θ§![]() Θ§
Θ§![]() )
) ![]() ΓΝ10-10
ΓΝ10-10
ΓΨΫβΈωΓΩ
(1)Ga «31Κ≈‘ΣΥΊΘ§‘≠Ή”ΚΥΆβ”–31ΗωΒγΉ”Θ§ΗυΨίΙΙ‘λ‘≠άμΩ…÷ΣΜυΧ§Ga‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ Ϋ «1s22s22p63s23p63d104s24p1Θ§ΜρΦρ–¥ΈΣ[Ar]3d104s24p1ΘΜ
(2)ΒΣΜ·≈πΓΔΨßΧεΙηΕΦ «‘≠Ή”ΨßΧεΘ§Τδ”≤Ε»”κ‘≠Ή”ΑκΨΕΓΔΦϋ≥Λ≥…Ζ¥±»ΓΘ”…”ΎN‘≠Ή”ΚΆB‘≠Ή”ΒΡΑκΨΕ±»Ιη‘≠Ή”–ΓΘ§B-NΦϋ≥Λ±»Si-SiΕΧΘ§B-NΦϋΒΡΦϋΡή±»Si-SiΦϋΒΡΦϋΡή¥σΘ§Υυ“‘ΒΣΜ·≈π(BN)±»ΨßΧεΙηΨΏ”–ΗϋΗΏ”≤Ε»ΚΆΡΆ»»–‘ΘΜ
(3)‘ΎBF3Ζ÷Ή”÷–B‘≠Ή”ΒΡΦέ≤ψΒγΉ”Ε‘Β»”Ύ3Θ§«“ΟΜ”–Ι¬ΒγΉ”Ε‘Θ§Υυ“‘÷––Ρ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά «sp2‘”Μ·ΘΜB‘≠Ή”ΉνΆβΒγΉ”≤ψΈΣL≤ψΘ§ΈόdΙλΒάΘ§ΕχSi‘≠Ή”ΉνΆβ≤ψΈΣM≤ψΘ§”–dΙλΒάΘ§Ω…≤Έ”κ‘”Μ·Θ§ ΙSi≈δΈΜ ΐ‘ωΦ”÷Ν6ΘΜ
(4)NaBH4 «άκΉ”Μ·ΚœΈοΘ§Na+”κBH4-Ά®ΙΐάκΉ”ΦϋΫαΚœΘ§BH4-÷–B”κHΆ®ΙΐΙ≤ΦέΦϋΫαΚœΘ§Υυ“‘NaBH4ΒΡΒγΉ” ΫΈΣΘΚ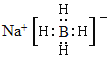 ΘΜ‘ΎNaBH4÷–Κ§”–NaΓΔBΓΔH»ΐ÷÷‘ΣΥΊΘ§”…”Ύ‘ΣΥΊΒΡΖ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΤδΒγΗΚ–‘ΨΆ‘Ϋ¥σΘ§‘ΣΥΊΒΡΖ«Ϋπ τ–‘ΘΚHΘΨBΘΨNaΘ§‘ρ‘ΣΥΊΒΡΒγΗΚ–‘ΘΚHΘΨBΘΨNaΘΜ
ΘΜ‘ΎNaBH4÷–Κ§”–NaΓΔBΓΔH»ΐ÷÷‘ΣΥΊΘ§”…”Ύ‘ΣΥΊΒΡΖ«Ϋπ τ–‘‘Ϋ«ΩΘ§ΤδΒγΗΚ–‘ΨΆ‘Ϋ¥σΘ§‘ΣΥΊΒΡΖ«Ϋπ τ–‘ΘΚHΘΨBΘΨNaΘ§‘ρ‘ΣΥΊΒΡΒγΗΚ–‘ΘΚHΘΨBΘΨNaΘΜ
(5)ΗΟΨßΑϊ÷–“θ―τάκΉ”÷°Φδ¥φ‘ΎάκΉ”ΦϋΘ§BΚΆO‘≠Ή”÷°Φδ¥φ‘ΎΙ≤ΦέΦϋΚΆ≈δΈΜΦϋΘ§Υ°Ζ÷Ή”÷°Φδ¥φ‘ΎΖ÷Ή”ΦδΉς”ΟΝΠ(ΜρΖΕΒ¬ΜΣΝΠ)ΚΆ«βΦϋΘ§Υυ“‘ΗΟΈο÷ ÷–≤ΜΚ§Ϋπ τΦϋΘ§Ι Κœάμ―Γœν «DΘΜ
(6)ΗυΨίΨßΑϊΫαΙΙΩ…÷ΣΘΚΨßΑϊ÷–B‘≠Ή”¥Π”ΎΨßΑϊΕΞΒψ”κΟφ–Ρ…œΘ§B‘≠Ή”ΈΣΟφ–ΡΝΔΖΫΉνΟήΕ―ΜΐΘ§ΨßΑϊ÷–BΓΔP‘≠Ή”Ηω ΐ±»ΈΣ1ΘΚ1Θ§P‘≠Ή””κ÷ήΈßΒΡ4ΗωB‘≠Ή”–Έ≥…ΒΡ «’ΐΥΡΟφΧεΫαΙΙΘ§P‘≠Ή”¥Π”Ύ’ΐΥΡΟφΧεΒΡ÷––ΡΘ§ΗυΨίΆΦ ΨΉχ±ξœΒΩ…÷ΣN¥ΠΒΡP‘≠Ή”Ζ÷ ΐΉχ±ξΈΣ(![]() Θ§
Θ§![]() Θ§
Θ§![]() )ΘΜP‘≠Ή””κ÷ήΈßΒΡ4ΗωB‘≠Ή”ΉνΫϋ«“–Έ≥…’ΐΥΡΟφΧεΫαΙΙΘ§Εΰ’ΏΝ§œΏ¥Π”ΎΧεΕ‘Ϋ«œΏ…œΘ§ΈΣΧεΕ‘Ϋ«œΏΒΡ
)ΘΜP‘≠Ή””κ÷ήΈßΒΡ4ΗωB‘≠Ή”ΉνΫϋ«“–Έ≥…’ΐΥΡΟφΧεΫαΙΙΘ§Εΰ’ΏΝ§œΏ¥Π”ΎΧεΕ‘Ϋ«œΏ…œΘ§ΈΣΧεΕ‘Ϋ«œΏΒΡ![]() Θ§≈π‘≠Ή”ΚΆΝΉ‘≠Ή”÷°ΦδΒΡΉνΫϋΚΥΦδΨύΈΣa pmΘ§‘ρΝΔΖΫΧεΕ‘Ϋ«œΏΈΣ4a pmΘ§ΦΌ…ηΝΔΖΫΧεΒΡ±Ώ≥ΛΈΣx pmΘ§‘ρ
Θ§≈π‘≠Ή”ΚΆΝΉ‘≠Ή”÷°ΦδΒΡΉνΫϋΚΥΦδΨύΈΣa pmΘ§‘ρΝΔΖΫΧεΕ‘Ϋ«œΏΈΣ4a pmΘ§ΦΌ…ηΝΔΖΫΧεΒΡ±Ώ≥ΛΈΣx pmΘ§‘ρ![]() x=4a pmΘ§Υυ“‘x=
x=4a pmΘ§Υυ“‘x=![]() =
=![]() ΓΝ10-10 cmΓΘ
ΓΝ10-10 cmΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ”…ΦΗ÷÷άκΉ”Μ·ΚœΈοΉι≥…ΒΡΜλΚœΈο÷–Κ§”–“‘œ¬άκΉ”÷–ΒΡ»τΗ…÷÷ΘΚKΘΪΓΔClΘ≠ΓΔNH![]() ΓΔMg2ΘΪΓΔBa2ΘΪΓΔCO
ΓΔMg2ΘΪΓΔBa2ΘΪΓΔCO![]() ΓΔSO
ΓΔSO![]() ΓΘΫΪΗΟΜλΚœΈο»ή”ΎΥ°ΚσΒΟ≥Έ«ε»ή“ΚΘ§œ÷»Γ»ΐΖί100 mLΗΟ»ή“ΚΖ÷±πΫχ––»γœ¬ Β―ιΓΘ
ΓΘΫΪΗΟΜλΚœΈο»ή”ΎΥ°ΚσΒΟ≥Έ«ε»ή“ΚΘ§œ÷»Γ»ΐΖί100 mLΗΟ»ή“ΚΖ÷±πΫχ––»γœ¬ Β―ιΓΘ
Β―ι–ρΚ≈ | Β―ιΡΎ»ί | Β―ιΫαΙϊ |
1 | Φ”»κAgNO3»ή“Κ | ”–ΑΉ…Ϊ≥ΝΒμ…ζ≥… |
2 | Φ”»κΉψΝΩNaOH»ή“Κ≤ΔΦ”»» | ’Φ·ΒΫΤχΧε1.12 L(±ξΉΦΉ¥Ωωœ¬) |
3 | Φ”»κΉψΝΩBaCl2»ή“ΚΘ§Ε‘ΥυΒΟ≥ΝΒμΫχ––œ¥Β”ΓΔΗ…‘οΓΔ≥ΤΝΩΘΜ‘Όœρ≥ΝΒμ÷–Φ”»κΉψΝΩœΓ―ΈΥαΘ§»ΜΚσΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΓΔ≥ΤΝΩ | ΒΎ“Μ¥Έ≥ΤΝΩΕΝ ΐΈΣ6.27 gΘ§ΒΎΕΰ¥Έ≥ΤΝΩΕΝ ΐΈΣ2.33 g |
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΗυΨί Β―ι1Ε‘ClΘ≠ «Ζώ¥φ‘ΎΒΡ≈–Εœ «________(ΧνΓΑ“ΜΕ®¥φ‘ΎΓ±ΓΑ“ΜΕ®≤Μ¥φ‘ΎΓ±ΜρΓΑ≤ΜΡή»ΖΕ®Γ±)ΘΜΗυΨί Β―ι1ΓΪ3≈–Εœ‘≠ΜλΚœΈο÷–“ΜΕ®≤Μ¥φ‘ΎΒΡάκΉ” «________ΓΘ
Θ®2Θ© ‘»ΖΕ®100 mL»ή“Κ÷–“ΜΕ®¥φ‘ΎΒΡ“θάκΉ”ΦΑΤδΈο÷ ΒΡΝΩ≈®Ε»(Ω…≤ΜΧν¬ζ)ΓΘ
“θάκΉ”ΖϊΚ≈ | Έο÷ ΒΡΝΩ≈®Ε»(molΓΛLΘ≠1) |
_______ | ___________ |
______ | _______________ |
Θ®3Θ©KΘΪ «Ζώ¥φ‘ΎΘΩ________(ΧνΓΑ¥φ‘ΎΓ±ΜρΓΑ≤Μ¥φ‘ΎΓ±)Θ§≈–ΕœΒΡάμ”… «____________________ΓΘ
ΓΨΧβΡΩΓΩΧΫΨΩ¬ΝΤ§”κNa2CO3»ή“ΚΒΡΖ¥”ΠΘΚ
| | |
ΈόΟςœ‘œ÷œσ | ¬ΝΤ§±μΟφ≤ζ…ζœΗ–ΓΤχ≈ί | ≥ωœ÷ΑΉ…ΪΜκΉ«Θ§≤ζ…ζ¥σΝΩΤχ≈ί(Ψ≠Φλ―ιΈΣ H2ΚΆCO2) |
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «( )
A.Na2CO3»ή“Κ÷–¥φ‘ΎΥ°ΫβΤΫΚβΘΚCO32-+2H2O![]() H2CO3+2OH-
H2CO3+2OH-
B.ΆΤ≤β≥ωœ÷ΑΉ…ΪΜκΉ«ΒΡ‘≠“ρΘΚAlO2-+HCO3-+H2O=Al(OH)3Γΐ+CO2Γϋ
C.Ε‘±»ΔώΓΔΔσΘ§ΥΒΟς Na2CO3»ή“ΚΡήΤΤΜΒ¬Ν±μΟφΒΡ±ΘΜΛΡΛ
D.Φ”»»ΚΆH2“ί≥ωΕ‘CO32-Υ°ΫβΤΫΚβ“ΤΕ·ΖΫœρΒΡ”Αœλ «œύΖ¥ΒΡ