��Ŀ����
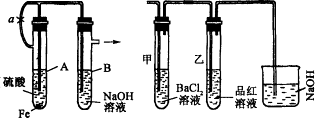
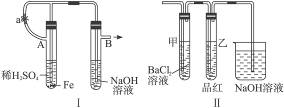
��������ʵ��װ�úͲ������ش��й����⣺(1)��ͼ������ʾװ�ã������ֲ�ͬ�����ֱ����ʵ�飬�۲�B��������
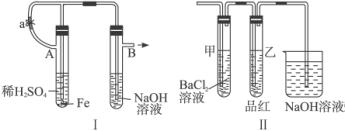
����1���ȼн�ֹˮ��a����ʹA�ܿ�ʼ��Ӧ��ʵ������B���й۲쵽��������____________________________________________________________________��
B���з�����Ӧ�����ӷ���ʽ��______________________________________________��
����2����ֹˮ��a��ʹA�ܿ�ʼ��Ӧһ��ʱ����ټн�ֹˮ��a��ʵ������B���й۲쵽��������________________________________________________________________��
(2)����װ�õ�ʵ�������ȡA���ڷ�Ӧ������Һ����������С�����ɺ��ٸ������գ��й�װ�ü���������ȥ����������º�ɫ���塣���ֽ�ʱ���������尴ͼ����ʾװ������ͨ��ϴ��װ�ã�����Թܼ��ڳ��ְ�ɫ�������Թ�������Һ��ɫ��ɫ���ش�
���û�ѧ����ʽ˵���Թܼײ�����ɫ������ԭ��������˵����____________________��
�ڸ���ʵ������д��ͼ����A������Һ���ɺ��ڸ��������շֽ�ʱ��������������ԭ��Ӧ�Ļ�ѧ����ʽ_____________________________________________________��
�����Ӧ����������_______________________����ԭ����____________________________��
(1)����1���ȳ��ְ�ɫ��״������Ȼ�����Ѹ�ٱ����ɫ������Ϊ���ɫ
Fe2++2OH-====Fe(OH)2�� 4Fe(OH)2+O2+2H2O====4Fe(OH)3
����2����ֹˮ��ʱ����Һ�еĵ��ܿ�������ð�����н����Ӻ��ְ�ɫ��״������һ��ʱ���ڳ�������ɫ
��2����SO3+BaCl2+H2O====BaSO4��+2HCl
��2FeSO4![]() Fe2O3+SO2��+SO3�� FeSO4 FeSO4
Fe2O3+SO2��+SO3�� FeSO4 FeSO4
�����������漰��϶࣬�ȿ���ѧ����ʵ���������ֿ���˼ά�������ԡ������Ժʹ����ԡ������һ������Χ��Fe(OH)2��ʵ�����Ʒ���Ƶġ�����Fe(OH)2���ױ�����Ϊ?Fe(OH)3����Һ���ܽ��O2���ܰ�Fe(OH)2���������н�aʱ��A�Թܱ����һ�������ϵ��������H2ʹA�Թ���ѹǿ�������ɵ�FeSO4��Һѹ��B�У��Ӷ�������Ӧ����?Fe(OH)2���ɡ���NaOH��Һ���ܽ��O2�ɽ�Fe(OH)2Ѹ�����������ת����Fe(OH)3�����ԣ�������ɫ������ʱ��̡ܶ������в���2ʱ��������H2����ͨ��������ͨ�뵽B��NaOH��Һ�У��������ܽ��O2���ߣ��ټн�aʱ��FeSO4��Һѹ��B�У���ʱ��Ӧ��������Fe(OH)2������һ��ʱ���ڲ���ɫ������ڶ�����Χ��FeSO4��������ǿ��ʱ�IJ���������������������ѧ����������ԭ֪ʶ�ƶϳ�FeSO4���·ֽⷴӦ�Ļ�ѧ����ʽ��