��Ŀ����
���Ṥҵβ���еĵ���������(NO��NO2)����Ҫ�Ĵ�����Ⱦ��䳣�õ�������������������(�Ѽ�)
��NaOH���շ���Ӧԭ�����£�2 NO2+2NaOH=NaNO3+NaNO2+H2O��NO+NO2+2NaOH=2NaNO2+ H2O
�ڰ�����ԭ�� ��Ӧԭ���ǣ�NO2+NH3��N2+H2O
����һ�����ĺ�NO2��NO�����Ṥҵβ��(������������)�����ù�����NaOH��Һ���պ���Һ�� NaNO3�� NaNO2�����ʵ���֮��ǡ����β����NO�� NO2�����ʵ���֮����ȡ�
(1)����NOx��ʾ��β���е����������ƽ����ɣ�����x��ֵ��
(2)��1����ĸ�β���ð�����ԭ������������������ͬ״���¶�������İ���?
(1)x��ֵΪ7��4��(2)1�����β������������ͬ״����7��6����İ�����
����:
(1)���������� NO2�����ʵ���a��NO�����ʵ���Ϊb
2 NO2+2NaOH=NaNO3+NaNO2+H2O
a��b 0��5(a��6) 0��5(aһ6)
NO+NO2+2NaOH=2NaNO2+ H2O
b b b
��������a��b=3��l
��ʮ�ֽ��淨��x��ֵ(��xֵ������NO2�� NO����ԭ������ƽ��ֵ)
�����İ������Ϊl+1��6=7��6(���)��

 )����Ҫ�Ĵ�������䳣�õ�������������������(�Ѿ���)��
)����Ҫ�Ĵ�������䳣�õ�������������������(�Ѿ���)��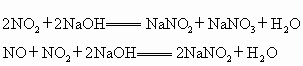

 ��
��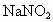 �����ʵ���֮��ǡ����β����NO��
�����ʵ���֮��ǡ����β����NO�� ��ʾ��β���е����������ƽ����ɣ�����xֵ��
��ʾ��β���е����������ƽ����ɣ�����xֵ��