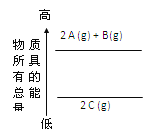
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷Σœ¬Ν–»»Μ·―ßΖΫ≥Χ ΫΘΚ
ΔΌ H2(g)+![]() O2(g)ΘΫH2O(l) ΠΛH ΘΫ®D285kJ/mol
O2(g)ΘΫH2O(l) ΠΛH ΘΫ®D285kJ/mol
ΔΎ H2(g)+![]() O2(g)ΘΫH2O(g) ΠΛH ΘΫ®D241.8kJ/mol
O2(g)ΘΫH2O(g) ΠΛH ΘΫ®D241.8kJ/mol
Δέ C(s)+![]() O2(g)ΘΫCO(g) ΠΛH ΘΫ®D110.4 kJ/mol
O2(g)ΘΫCO(g) ΠΛH ΘΫ®D110.4 kJ/mol
Δή C(s)+ O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D393.5 kJ/mol
ΜΊ¥πœ¬Ν–ΗςΈ ΘΚ
Θ®1Θ©…œ ωΖ¥”Π÷– τ”ΎΖ≈»»Ζ¥”ΠΒΡ «___________ΓΘ
Θ®2Θ©CΒΡ»Φ…’»»ΈΣ_________________ΓΘ
Θ®3Θ©»Φ…’10g H2…ζ≥…“ΚΧ§Υ°Θ§Ζ≈≥ωΒΡ»»ΝΩΈΣ_____________________ΓΘ
Θ®4Θ©CO»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ______________________ΓΘ
Θ®5Θ©ΈΣΝΥΩΊ÷ΤΈ¬ “–ß”ΠΘ§ΗςΙζΩΤ―ßΦ“Χα≥ωΝΥ≤Μ…Ό…ηœκΓΘ”–»ΥΗυΨί“ΚΧ§CO2ΟήΕ»¥σ”ΎΚΘΥ°ΟήΕ»ΒΡ ¬ ΒΘ§…ηœκΫΪCO2“ΚΜ·ΚσΘ§ΥΆ»κ…νΚΘΚΘΒΉΘ§“‘Φθ…Ό¥σΤχ÷–ΒΡCO2ΓΘΈΣ ΙCO2“ΚΜ·Θ§Ω…≤…»ΓΒΡ¥κ ©________ΓΘ
a Φθ―ΙΓΔ…ΐΈ¬ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ b ‘ω―ΙΓΔΫΒΈ¬
c Φθ―ΙΓΔΫΒΈ¬ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ d ‘ω―ΙΓΔ…ΐΈ¬
ΓΨ¥πΑΗΓΩΔΌΔΎΔέΔήΘΜ 393.5 kJ/molΘΜ 1425 kJ CO(g)+![]() O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D283.1kJ/molΘΜ b
O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D283.1kJ/molΘΜ b
ΓΨΫβΈωΓΩ
Θ®1Θ©Ζ≈»»Ζ¥”ΠΒΡΠΛH<0ΘΜ
Θ®2Θ©»Φ…’»»ΘΚ1 mol¥ΩΈο÷ Άξ»Ϊ»Φ…’…ζ≥…Έ»Ε®ΒΡ―θΜ·Έο ±ΥυΖ≈≥ωΒΡ»»ΝΩΘΜ
Θ®3Θ©ΗυΨίΈο÷ ΒΡΝΩ±Ε ΐΙΊœΒΦΤΥψΖ≈≥ωΒΡ»»ΝΩΘΜ
Θ®4Θ©”…Η«ΥΙΕ®¬…ΦΤΥψΘΜ
Θ®5Θ©Φ”―ΙΫΒΈ¬Ω…Φθ–ΓΖ÷Ή”÷°ΦδΒΡΦδΗτΘ§ ΙΕΰ―θΜ·ΧΦ”…ΤχΧε±δΈΣ“ΚΧεΓΘ
Θ®1Θ©Ζ≈»»Ζ¥”ΠΒΡΠΛH<0Θ§Ι ΔΌΔΎΔέΔήΕΦ «Ζ≈»»Ζ¥”ΠΘΜ
Θ®2Θ©1 mol¥ΩΈο÷ Άξ»Ϊ»Φ…’…ζ≥…Έ»Ε®ΒΡ―θΜ·Έο ±ΥυΖ≈≥ωΒΡ»»ΝΩΈΣ»Φ…’»»Θ§‘ρΖ¥”ΠΔή÷–CΆξ»Ϊ»Φ…’Θ§CΒΡ»Φ…’»»ΈΣ393.5 kJ/molΘΜ
Θ®3Θ©»Φ…’10g H2Φ¥5mol H2…ζ≥…“ΚΧ§Υ°Θ§Ζ≈≥ωΒΡ»»ΝΩΈΣ285kJ![]() 5=1425 kJΘΜ
5=1425 kJΘΜ
Θ®4Θ©”…ΔέC(s)+![]() O2(g)ΘΫCO(g) ΠΛH ΘΫ®D110.4 kJ/molΘ§ΔήC(s)+ O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D393.5 kJ/molΘ§Δή-ΔέΩ…ΒΟCO»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣCO(g)+
O2(g)ΘΫCO(g) ΠΛH ΘΫ®D110.4 kJ/molΘ§ΔήC(s)+ O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D393.5 kJ/molΘ§Δή-ΔέΩ…ΒΟCO»Φ…’ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣCO(g)+![]() O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D283.1kJ/molΘΜ
O2(g)ΘΫCO2(g) ΠΛH ΘΫ®D283.1kJ/molΘΜ
Θ®5Θ©”…Έο÷ ΒΡ»ΐΧ§±δΜ·Ω…÷ΣΘ§‘Ύ¥σΤχΧθΦΰœ¬Θ§Εΰ―θΜ·ΧΦ «Έό…ΪΈόΈΕΒΡΤχΧεΘ§Εχ‘ΎΈ¬Ε»ΒΆ”Ύ31.2Γφ ±Θ§Φ”―ΙΩ… ΙCO2±δΈΣ“ΚΧ§Θ§Φ¥Φ”―ΙΫΒΈ¬Ω…Φθ–ΓΖ÷Ή”÷°ΦδΒΡΦδΗτΘ§ ΙΕΰ―θΜ·ΧΦ”…ΤχΧε±δΈΣ“ΚΧεΘ§‘ρΩ…ΫΪCO2“ΚΜ·ΚσΥΆ»κ…νΚΘΚΘΒΉΘ§“‘Φθ–Γ¥σΤχ÷–CO2ΒΡ≈®Ε»Θ§¥πΑΗ―ΓbΓΘ

ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ»ΐΗω»ίΜΐœύΆ§ΒΡΚψ»ίΟή±’»ίΤς÷–Α¥≤ΜΆ§ΖΫ ΫΆΕ»κΖ¥”ΠΈοΘ§ΖΔ…ζΖ¥”Π 2SO2(g) + O2(g)![]() 2SO3(g)(’ΐΖ¥”ΠΖ≈»»)Θ§œύΙΊ ΐΨί»γœ¬Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
2SO3(g)(’ΐΖ¥”ΠΖ≈»»)Θ§œύΙΊ ΐΨί»γœ¬Θ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
»ίΤς 1 | »ίΤς 2 | »ίΤς 3 | |
Ζ¥”ΠΈ¬Ε» T/K | 700 | 700 | 800 |
Ζ¥”ΠΈοΆΕ»κΝΩ | 2molSO2ΓΔ 1molO2 | 4molSO3 | 2molSO2ΓΔ 1molO2 |
ΤΫΚβΠΆ’ΐ(SO2 )/molΓΛL-1ΓΛs-1 | ΠΆ1 | ΠΆ2 | ΠΆ3 |
ΤΫΚβ c(SO3 )/ molΓΛL-1 | c1 | c2 | c3 |
ΤΫΚβΧεœΒΉή―Ι«Ω p/Pa | p1 | p2 | p3 |
Έο÷ ΒΡΤΫΚβΉΣΜ·¬ ®Μ | ΠΝ1(SO2) | ΠΝ2(SO3) | ΠΝ3(SO2) |
ΤΫΚβ≥Θ ΐ K | K1 | K2 | K3 |
A. ΠΆ1<ΠΆ2Θ§c2<2c1 B. K1>K3Θ§p2>2p3
C. ΠΆ1< ΠΆ3Θ§ΠΝ1(SO2) > ΠΝ3(SO2) D. c2>2c3Θ§ΠΝ2(SO3) +ΠΝ3(SO2)<1